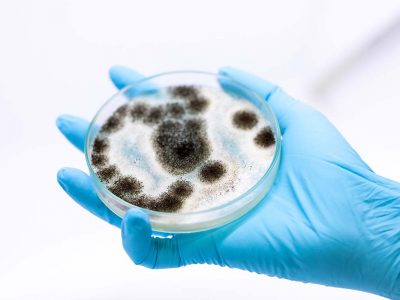
Healthcare mold management. It’s a concern for an increasing number of organizations. And if you read the newspapers, you have likely seen the reports from a hospital in Seattle where environmental mold was linked to some patient infections. For at least two years, The Joint Commission has indicated that mold is taking on an increasing importance in the survey process. And a recent EC News contains an excellent article from about mold prevention and abatement.
Healthcare Mold Management
The article discusses changes made to EC.02.05.01, EP 15. This EP discusses airborne contaminants, temperature, and air pressure relationships in critical areas. The EP changed and now requires compliance with ASHRAE 170 2008, or state requirements if more stringent.
The ASHRAE standard establishes expectations for air filtration efficiency called MERV. It uses a 1 – 20 scale with higher numbers indicating greater filtration. Specifically, they advise surgical settings to have a MERV of at least 14 for the second filter bank. Further, areas serving immunocompromised patients should have a MERV of at least 17 for the second filter bank. Ratings of 17 and above are considered HEPA filtration.
In addition to the adequacy of filtration, there will also be a survey focus on maintaining filtration system to the manufacturers specifications, or MIFU. This will include guidance on the type of filter and the frequency of changing these filters. Also, you must gasket these filters in place. Lastly, if you are changing filters, you can’t use the operating rooms unless there is redundant equally effective filtration.
The key point here is the frequent scoring of EP 15. This is an ongoing problem area due to challenges with temperature, humidity, and air pressure. And, given the increasing complexity of the requirement, scoring will likely increase.
EP Note Additions
There are also two new notes added to this EP. The first expresses that hospitals may implement a CMS categorical waiver to permit humidity as low as 20% in operating rooms. But, the note also states that if you do this you must verify that your equipment and supplies are compatible with this lower humidity level. Getting such evidence from manufacturers is no easy task.
Note two requires hospitals who use accreditation for deemed status purposes to follow one of two actions:
- Comply with NFPA 99-2012 ventilation requirements
- Follow the ventilation requirements adopted by CMS at the time of installation
Proper air pressure relationships, air exchanges, dust prevention, and humidity levels can help reduce the possibility of mold formation. Unfortunately, dust formation during construction and renovation can contribute to problems. So can the ever-problematic water leaks that occur.
The authors tie back in the importance of well-designed and fully implemented ICRA and PCRA plans. This helps prevent dust particles from escaping from the construction area. In fact, the authors point to the 2018 FGI Guidelines. They contain new infection control risk mitigation recommendations (ICRMRs) and the CDC’s Environmental Infection Control in Health Care Facilities Guideline, updated in July 2019. TJC then highlights some of these recommendations. One is maintaining the negative pressure value in the construction zone at least 0.03-inch water column to ensure adequate airflow out of the construction.
Given the risk to patients from mold, and the potential survey emphasis, this is an important article to share with your infection prevention and environment of care committees. But make sure you send as a question not as an FYI. And the question is, are we already adherent to these recommendations or do we need to do something more?
Infection Prevention and Control Issues in the Environment of Care
Later on in the February EC News, there is an article/advertisement for a new book by JCR on Infection Prevention and Control Issues in the Environment of Care. We don’t usually discuss advertisements. But this links to a companion case study. It describes a real-world nightmare at the New York Presbyterian Allen Hospital. A main sanitary line broke causing a waste backup in the surgical areas. The line repair had to occur under the semi-sterile corridor.
The authors describe the very significant scope of their problem. They also detail the measures they took to safely manage this repair and the ICRA measures they undertook in doing so. It is a good piece to share in the context of our earlier discussion about mold abatement and ICRA in general.

 Closed Loop Communication Of Test Results
Closed Loop Communication Of Test Results
Leave a Reply