October 2022
Inside This Issue
EC and LS Standards: New and Changes: 
This month’s edition of Perspectives has a brief article on new EC and LS standards changes that become effective January 1, 2023. Usually, unless CMS has mandated some immediate change, TJC provides a minimum of six months’ notice.
Upon closer inspection however, for the most part, these don’t appear to be new requirements, but rather more explanatory notes and wording changes to existing elements of performance. There are ten pages of changes with two EC standard edits and eight LS standard edits. All should be carefully reviewed as you may have to change some existing language in policies or plans, but for the most part not have to start some entirely new process.
We only noted two entirely new EPs under LS.05.01.34 which apply in business occupancies. Although not stated in the article, our assumption is that the relatively minor wording changes are designed to keep the language in the elements of performance aligned with the appropriate and up to date NFPA manuals. The prepublication version provides the existing 2022 text of the EP, along with the modified text of the 2023 EPs.
Do print this off and share with your facilities leadership, as this side-by-side comparison of new and existing language is helpful to seeing the subtle differences in the modified EPs. If you just look at your 2023 manuals or E-edition it will be too easy to miss some of these changes.
One change that adds clarity to an issue that has been confusing is the modification to EC.01.01.01, EP 9 that requires a plan for managing utility systems. The part that has been confusing is – what does the organization need to do in a business occupancy that is rented space, not owned by the organization? The new EP states that the “plan only needs to address how routine service and maintenance for their utility systems are obtained.”
A second explanatory change was noted in EC.02.05.01, EP 23 regarding power strips in the patient care “vicinity.” The modified EP adds details how the term patient care “vicinity” is defined in NFPA 99-2012 as up to 1.8 meters from the patient and 2.3 meters above the floor.
We also noted a change in LS.05.01.10, EP 5 for business occupancies, which currently requires the space around pipes, cables, air ducts, etc., that penetrate walls or floors to be fire rated. The modified language seems to set some limitations around the use of fire rated sealant, stating the requirement is limited to “fire rated walls and floors.”
Lastly, the new EPs 4 and 5 are under LS.05.01.34. EP 4 discusses the need to have a process for emergency response notification in new construction. EP 5 then discusses existing construction sites who replace their fire alarm systems need to build in this emergency notification process in their new fire alarm system.
Definition of Sexual Abuse/Assault:
Perspectives announces a change to the TJC definition of sexual abuse/assault. The revised and expanded definition includes content from the CMS State Operations Manual for Long Term Care Facilities, also known as Appendix PP. We were initially surprised at the reference to the long-term care state operations manual, but then we did a term search for sexual abuse in both the CMS hospital and long-term care manuals and the content discussion in the LTC manual is far more robust.
If you download these CMS documents as PDFs you can use the search box to find references to sexual, sexual abuse, sexual assault and you will note the much richer discussion in the LTC manual. In particular see sections F540 and F600 in the LTC manual.
We have not restated the revised definition here because as a publisher we need to avoid extensive verbatim quotes of TJC material to avoid potential copyright infringement. The revised definition is quite extensive. The key point we need to make on this issue is that prior to January 1, 2023 you will want to update any existing definition of sexual abuse/assault you may have in your current sentinel event policy.
In addition, be sure to update the headers on that policy to make it clear it was updated in 2022. If a surveyor asks to see your Sentinel Event Policy, you make an RFI too easy to identify if your policy was last updated more than a year ago. Remember also that managing and analyzing sentinel events is mandatory, whereas it is only reporting to TJC is optional. Thus, your definitions of what is or is not sentinel should align with TJC’s.
Proficiency Testing Regulations for Laboratories: 
Perspectives discusses a posting CMS made in the Federal Register July 11, 2022 that affect proficiency testing. One portion of these new CMS requirements took place in August and additional changes become effective in 2024. The immediate change made in August relative to proficiency testing performed in moderate or high complexity laboratories will cause TJC to score an existing standard, QSA.01.04.01 in the laboratory manual noncompliant.
TJC will be making revisions to their laboratory standards to accommodate the additional changes CMS announced. To assist with planning for these changes and understanding the immediate change we have copied the link to the Federal Register announcement from CMS. https://www.govinfo.gov/content/pkg/FR-2022-07-11/pdf/2022-14513.pdf
Medical Air vs. Instrument Air:
This month’s Consistent Interpretation column discusses an issue what is somewhat poorly understood in healthcare and that is the differences between medical air and instrument air. Medical grade air is intended for breathing, is required to maintain a certain dew point and must include monitoring and alarming for carbon monoxide. Instrument air is specially filtered and dried.
The particular EP scored for this issue is EC.02.05.09, EP 14, which is a somewhat generic EP requiring compliance with NFPA 99 related to gas and vacuum systems. TJC reports this EP as scored in roughly 7% of hospitals last year.
The key difference in these two types of air, is that medical air is intended for use in humans and it is a clean, oil free form of air with humidity and instrument air is also very pure but very dry. Instrument air is used to dry medical equipment often after high level disinfection.
Neither of these forms of air are interchangeable. Instrument air cannot be used on humans and medical air cannot be used on instruments. With the rapid proliferation of the number of sites performing high level disinfection we have also seen a third type of compressed air being used, which is just compressed room air provided by a generic home improvement store compressor.
While seemingly useful in a site that was not piped for instrument air, the home improvement store compressor does not actually provide the very dry and purified air needed for instrument processing.
Do share this consistent interpretation column with your facilities staff and staff performing high level disinfection to ensure the appropriate form of air is actually being used.
Decarbonization: 
This month’s edition includes a brief summary of the TJC Presidents keynote discussion at this year’s Executive Briefings session. It continues the discussion relative to TJC’s focus on climate change and decarbonization and references a planned comprehensive review of the standards to identify those that exceed CMS regulations.
We had an opportunity to participate in a similar analysis many years ago while working for TJC and while it was an interesting experience there were many constituencies that had strong opinions and advocated to retain many existing requirements, not related to CMS regulations or directly linked to patient safety. We wish them well in the next iteration of this process.
This EC News article also references a Decarbonization Technical Advisory Panel that TJC Is forming to review pertinent standards. For those of you that desire to get ahead of this curve we did note that AHRQ published a Decarbonization Primer this past month that you can download from: https://www.ahrq.gov/healthsystemsresearch/decarbonization/index.html
ED Access and Security: 
EC News also has a good article titled “Improving Emergency Department Access.” However, the article is not just focused on improved access, it also discusses improving security while improving access. The article discusses a new law in MA, called “Laura’s Law,” named after a young woman who suffered an asthma attack just outside the hospital after not being able to find an unlocked entrance to the emergency department.
The new MA law goes into effect January 1, 2024 and effects signage, wayfinding, security, and lighting. There is a boxed section within the article that also mentions that EMTALA requirements begin within 250 yards of the hospital, once a patient has reached the hospital campus or grounds.
The article looks like it would be good to review with ED staff/leaders at an environment of care committee. Topics discussed include access requirements, assessing risk including risk of workplace violence, use of cameras and alarms, wayfinding/signage and lighting and even digital navigation.
Asymptomatic Covid Testing:
There was only one new CMS QSO memo of interest to readers this past month and that was QSO-22-25, dated September 26th, 2022. CMS is rescinding what is called “enforcement discretion” for performing Covid testing on asymptomatic individuals in both waived and nonwaived laboratory settings. This type of testing is outside the scope of the test’s authorized performance specifications.
At home testing is widely available and thus laboratory settings should not have to perform such testing on asymptomatic individuals. You can access the CMS memo using this link: https://www.cms.gov/medicareprovider-enrollment-and-certificationsurveycertificationgeninfopolicy-and-memos-states-and/cms-rescinds-december-7-2020-enforcement-discretion-use-sars-cov-2-tests-asymptomatic-individuals
Covid Source Control for Healthcare Workers:
The CDC made news this past month when on September 23rd they updated their “Interim Infection Prevention and Control Recommendations for Healthcare Personnel During the Coronavirus Disease 2019 Pandemic.” This is a lengthy document that the CDC has been revising and updating throughout the pandemic as new information and guidance became available. You can find the entire posting here: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html
The section we wanted to bring to your attention deals with healthcare workers and source control or masks. The guidance is somewhat technical and precise; thus, we will quote the CDC verbatim rather than try to summarize this section.
“When SARS-CoV-2 Community Transmission levels are high, source control is recommended for everyone in a healthcare setting when they are in areas of the healthcare facility where they could encounter patients.
- HCP could choose not to wear source control when they are in well-defined areas that are restricted from patient access (e.g., staff meeting rooms) if they do not otherwise meet the criteria described below and Community Levels are not also high. When Community Levels are high, source control is recommended for everyone.
When SARS-CoV-2 Community Transmission levels are not high, healthcare facilities could choose not to require universal source control. However, even if source control is not universally required, it remains recommended for individuals in healthcare settings who:
- Have suspected or confirmed SARS-CoV-2 infection or other respiratory infection (e.g., those with runny nose, cough, sneeze); or
- Had close contact (patients and visitors) or a higher-risk exposure (HCP) with someone with SARS-CoV-2 infection, for 10 days after their exposure; or
- Reside or work on a unit or area of the facility experiencing a SARS-CoV-2 outbreak; universal use of source control could be discontinued as a mitigation measure once no new cases have been identified for 14 days; or
- Have otherwise had source control recommended by public health authorities”
“Individuals might also choose to continue using source control based on personal preference, informed by their perceived level of risk for infection based on their recent activities (e.g., attending crowded indoor gatherings with poor ventilation) and their potential for developing severe disease. For example, if an individual or someone in their household is at increased risk for severe disease, they should consider wearing masks or respirators that provide more protection because of better filtration and fit to reduce exposure and infection risk, even if source control is not otherwise required by the facility. HCP and healthcare facilities might also consider using or recommending source control when caring for patients who are moderately to severely immunocompromised.”
Now you might be wondering how do we determine if our community transmission rates are high or not. On the first page of the introduction in the previously mentioned memo, CMS discusses in a blue box section, the term “community transmission metric” which we should use in healthcare to help inform decision making on issues such as the use of masks for healthcare workers.
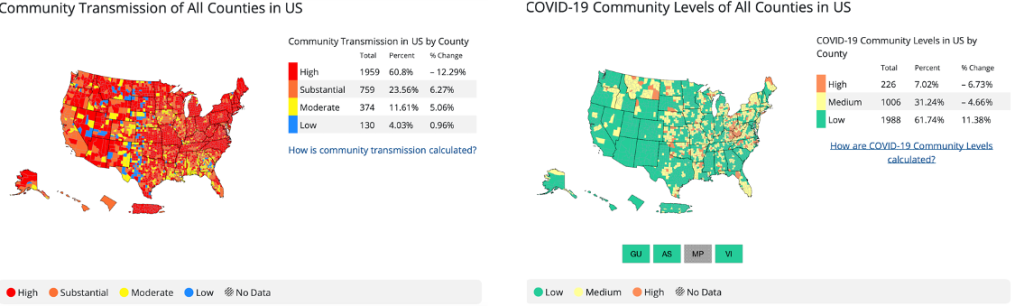
This is where things get tricky as there is also data on the CDC site called the “community level metric” which should only be used for non-healthcare settings. The community level metric looks at the rates of hospitalizations and strain on healthcare resources, not just positive cases.
We made this mistake in beginning to draft this newsletter when we printed the community level national map, which shows wide areas of the nation in green. When we realized our mistake and printed the community transmission map of the nation, it is mostly red with only a few counties in blue representing low transmission.
Note the chart below mostly in green is NOT the chart to use for decision making about universal source control for healthcare workers. The next chart, mostly in red, is the chart to use for the healthcare setting. In either chart you can zero in on your county’s specific data.
Consultant Corner
Dear Readers,
Well, we have somehow gotten to the last quarter of 2022 and there are plenty of new requirements to implement for the beginning of the year! The accrediting and regulatory agencies are still catching up on surveys due, and we don’t want you to be caught off guard when they show up.
As we enter the Holiday season, we are going to be celebrating the New Year before we even realize it. We are here for you before, during, and after survey to assist you in your compliance.
Please reach out to us today to get your mock or focus survey scheduled for 2023. Have a wonderful month!
Jennifer Cowel, RN MHSA
JenCowel@PattonHC.com
Kurt Patton, MS RPh
Kurt@PattonHC.com
John Rosing, MHA
JohnRosing@PattonHC.com
Mary Cesare-Murphy, PhD
MCM@PattonHC.com
