April 2021
Inside This Issue
Surveys are Restarting:
This month the major news is that both CMS and TJC have restarted on-site surveys. CMS has issued a QSO memo on the restart process which also addresses written follow-up requirements such as plans of correction for surveys already conducted but not yet closed out. We have already seen a few written summaries from organizations that have experienced an on-site 2021 TJC survey and in our TJC section below we discuss a few highlights that appeared to be a new focus of attention.
CAH Leadership Standards Changes:
There are three sets of standards changes announced in TJC’s Perspectives this month. The first change is in the leadership standards applicable to critical access hospitals only. The critical access hospital changes address the depth and breadth of the hospital’s performance improvement program, but there is nothing earth shattering here.
It looks more like someone at CMS perceived the TJC leadership standards as not being broad enough to cover the full scope of services, so TJC has tweaked the leadership chapter language to make it clear that the PI program must cover the full scope of services.
In our experience, the specifics in the PI chapter already makes this clear and that accredited critical access hospitals have already covered this base. CAHs should take a look at this revision, but it is likely you may not have to make any changes.

Full-Service Acute Hospital IM Standards Changes:
 For full service acute hospitals there is a new IM standard, IM.02.02.07, with five elements of performance that is intended to address the IT interoperability requirements, specifically 45 CFR 170.205(d)(2), which was an initiative that hospitals and EMR vendors were addressing in 2020.
For full service acute hospitals there is a new IM standard, IM.02.02.07, with five elements of performance that is intended to address the IT interoperability requirements, specifically 45 CFR 170.205(d)(2), which was an initiative that hospitals and EMR vendors were addressing in 2020.
These changes may spur surveyor discussions during one of the surveyor meetings with leadership so you will want information from your IT department about the status of implementation. Reading the new IM standard in isolation does not really make it clear what you are to do and who you are to notify.
To understand the new requirement in EPs 1-4, you will want to read both 45 CFR 170.205(d)(2) and the new elements of performance jointly. EP 5 in this standard set also addresses the ability to electronically send information to post discharge providers for coordination of care.
Water Management Requirements:
The third set of new standards is water management requirements and if you have been implementing the existing guidance from the CDC and ASHRAE on this subject, you are going to be well prepared. Conversely, if you have not implemented the guidance from CDC and ASHRAE, then these new standards may prove challenging.
 To a large extent, TJC has already been scoring these requirements using generic or catch-all EC and IC standards. While Perspectives briefly mentions the new requirements, EC News has a much more thorough discussion and we would encourage organizations to read the April edition of EC News for the detailed explanation.
To a large extent, TJC has already been scoring these requirements using generic or catch-all EC and IC standards. While Perspectives briefly mentions the new requirements, EC News has a much more thorough discussion and we would encourage organizations to read the April edition of EC News for the detailed explanation.
EC News also includes three references to an earlier Perspectives “Consistent Interpretation” column and EC News articles which add substantial knowledge about what is required. The consistent interpretation column from Perspectives in January 2020 was actually about the scoring already taking place for these new requirements using seven existing 2020 standards and ten elements of performance.
EC News referenced two earlier articles they wrote on this subject in December 2020 and February 2019. This month’s edition of EC News includes the prepublication version of the new requirements and an 8-page article explaining in detail the requirements and risks. We suggest your team review this most recent article, along with the three earlier ones mentioned previously.
There is one new water management standard, EC.02.05.02 with four elements of performance. In theory the implementation date is January 2022, however as we have mentioned and Perspectives has detailed, the essence of these requirements from the CDC and ASHRAE documents is already being scored. In addition, CMS had issued SC letter 17-30 almost four years ago establishing water management requirements in accordance with CDC guidance.
EP 1 requires the organization to appoint an individual or team to provide oversight and implement water management activities. The CDC provided this diagram detailing the skills and requirements for your team or leader.
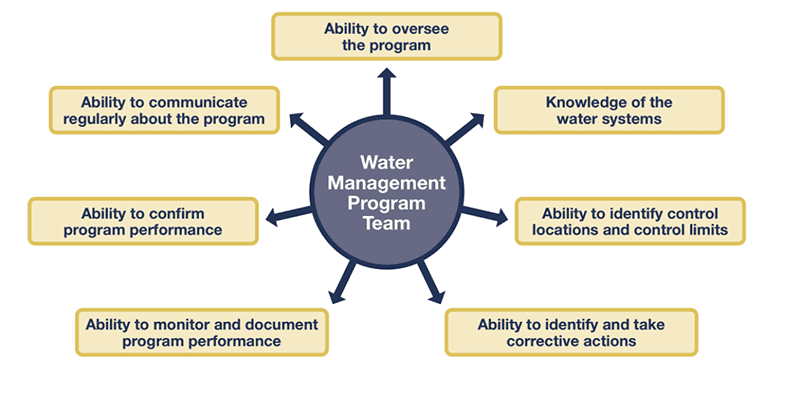 EP 2 details what the individual or team is supposed to do, and this includes:
EP 2 details what the individual or team is supposed to do, and this includes:
- Create a basic diagram that maps all water supply sources, treatment systems, processing steps, control measures, and end use points.
- Create a water risk management plan based on the diagram that includes an evaluation of the physical and chemical conditions of each step of the water flow diagram to identify any areas where potentially hazardous conditions may occur. (e.g., slow or stagnant water). TJC suggests here that organizations refer to the CDC’s Water Infection Control Risk Assessment (WICRA) for Healthcare Settings tool.
- A plan for addressing the use of water in areas of the building where water been stagnant for a period (e.g., during temporary closure).
- An evaluation of the patient populations served to identify patients who are immunocompromised.
- Monitoring protocols and acceptable ranges for control measures (e.g., temperatures, pH, disinfectant levels, locations to measure, and required corrective actions).
EP 3 requires the team or individual to manage:
- Documentation of all results of monitoring.
- Corrective actions and procedures if a test result is outside acceptable limits, including when a probable or confirmed waterborne pathogen indicates action is needed.
- Documenting corrective actions when control limits are not maintained.
 EP 4 requires an annual evaluation of the water management program and whenever the following occurs:
EP 4 requires an annual evaluation of the water management program and whenever the following occurs:
- Changes have been made to the water system that add risk.
- New equipment or at-risk water system has been added that would generate aerosols or be a potential source of legionella. This includes commissioning of a new wing or building.
Each of the new standard requirements can be downloaded from the Joint Commission’s Prepublication Standards Page.
Emergency Management Standards Guidance:
 Also, this month CMS issued QSO 21-15 which includes interpretive guidance for the new emergency management standards that were first published in the Federal Register as part of the Federal Burden reduction back in 2019, and in a QSO memo in early 2020, without interpretive guidance.
Also, this month CMS issued QSO 21-15 which includes interpretive guidance for the new emergency management standards that were first published in the Federal Register as part of the Federal Burden reduction back in 2019, and in a QSO memo in early 2020, without interpretive guidance.
Unfortunately, the memo addresses all types of inpatient and outpatient CMS providers so you have to make sure you are looking at requirements for your
provider type only. QSO 21-15 identifies the new language in red, which makes it easy to identify changes, however we would encourage readers to have their EM teams go through the entire document.
Each time we re-read it; we note content we had not previously identified. One key take away is that CMS advises inpatient providers that a minimum of 2 years of drill documentation will be reviewed, and outpatient providers, who only perform one drill a year will have 4 years of drill critiques reviewed.
Visitation Rights:
 The last high priority issue we want to highlight this month is that on March 10th CMS revised QSO 20-39 on visitation rights that had initially be published on September 17, 2020. This memo was addressed to the nursing home industry, but it is important for hospital readers also because of the CMS focus on patient rights.
The last high priority issue we want to highlight this month is that on March 10th CMS revised QSO 20-39 on visitation rights that had initially be published on September 17, 2020. This memo was addressed to the nursing home industry, but it is important for hospital readers also because of the CMS focus on patient rights.
Because of the continued expansion of the Covid-19 vaccination program, CMS is advising organizations to re-examine their policies on visitation, while maintaining infection prevention practices.
In this memo, CMS recommends outdoor visitation where feasible, but also states, “facilities should allow indoor visitation at all times and for all residents (regardless of vaccination status) except in a few circumstances when visitation should be limited due to a high risk of Covid-19 transmission (note: compassionate care visits should be permitted at all times).
These scenarios include limiting indoor visitation for:
- Unvaccinated residents if the nursing homes Covid-19 county positivity rate is >10% and <70% of residents are fully vaccinated.
- Residents with confirmed Covid-19 infection, whether vaccinated or unvaccinated until they have met the criteria to discontinue transmission-based precautions; or
- Residents in quarantine, whether vaccinated or unvaccinated, until they have met criteria for release from quarantine.”
 Another important point that CMS makes in this revised memo is that, “Federal and state surveyors are not required to be vaccinated and must be permitted entry into facilities unless they exhibit signs and symptoms of Covid-19. Surveyors should also adhere to the core principles of Covid-19 infection prevention, and adhere to Covid-19 infection prevention requirements set by state law.
Another important point that CMS makes in this revised memo is that, “Federal and state surveyors are not required to be vaccinated and must be permitted entry into facilities unless they exhibit signs and symptoms of Covid-19. Surveyors should also adhere to the core principles of Covid-19 infection prevention, and adhere to Covid-19 infection prevention requirements set by state law.
We see two important conclusions from this nursing home directive. The first is that we cannot continue restrictions on visitation if there is no longer an infection prevention necessity. Second, if CMS surveyors show up at your door, they will have to be permitted in to conduct their survey. We have seen guidance from TJC that they are taking the same position relative to their surveyors being permitted access to conduct survey activities.
Suite Aisle Width:
 This month’s edition of Perspectives has a very brief article indicating that TJC surveyors are starting to look for a minimum aisle width of 36 inches in suites. A suite we often describe as a series of rooms inside a larger room. Formal identification of a space as a suite is sometimes used in settings to permit hallway storage that would be prohibited in an exit corridor.
This month’s edition of Perspectives has a very brief article indicating that TJC surveyors are starting to look for a minimum aisle width of 36 inches in suites. A suite we often describe as a series of rooms inside a larger room. Formal identification of a space as a suite is sometimes used in settings to permit hallway storage that would be prohibited in an exit corridor.
However, it sounds as if TJC has noted what could best be described as excessive suite storage in the aisle that sometimes causes a restriction. A situation where the suite aisle is less than 36 inches will now be scored under LS.02.01.20. We would suggest taking a look at your common suite areas such as operating rooms and ICU halls to verify this minimum clearance.
Medical Assessments for Outpatient Procedures:
 The “Consistent Interpretation” column this month discusses the new option to allow a brief medical assessment in lieu of a full history and physical prior to selected outpatient procedures. This is particularly helpful because many organizations are still contemplating whether or not to pursue this option, and the article helps to explain what TJC is really expecting.
The “Consistent Interpretation” column this month discusses the new option to allow a brief medical assessment in lieu of a full history and physical prior to selected outpatient procedures. This is particularly helpful because many organizations are still contemplating whether or not to pursue this option, and the article helps to explain what TJC is really expecting.
There is not a lot of scoring history for this new option, so we recommend focusing on this Guidance/Interpretation column for each element of performance discussed. We suggest sharing this article with your medical staff leadership if this is something that they have been considering. The details presented in the Guidance may help them decide if they want to pursue this option and if so, how to authorize it in compliance with the new standards.
Maintaining Security Systems:
 After the very lengthy article on water management there is a brief article on maintaining security systems. A key point made by the authors is that security systems should be planned and not overbuilt. In other words, don’t install more than you can maintain appropriately. These devices require preventative maintenance and if you don’t have the staff to perform the required maintenance, you are in sense, replacing one risk for another.
After the very lengthy article on water management there is a brief article on maintaining security systems. A key point made by the authors is that security systems should be planned and not overbuilt. In other words, don’t install more than you can maintain appropriately. These devices require preventative maintenance and if you don’t have the staff to perform the required maintenance, you are in sense, replacing one risk for another.
The authors also caution that cameras get misaligned or misaimed, vegetation can grow in front of the camera, and dirt can accumulate making the visual worthless. There is also a helpful reference provided to NFPA 731 Standard for the Installation of Premises Security Systems, which provides guidance on inspection and testing frequencies.
BH Environment Most Frequently Scored:
 EC News also contains an article on the most frequently cited behavioral health environmental standards, but there really are only two that make it into the Top 10 of all standards scored.
EC News also contains an article on the most frequently cited behavioral health environmental standards, but there really are only two that make it into the Top 10 of all standards scored.
The first is the environmental aspect of suicide safety under NPSG.15.01.01, the 2nd most frequently scored standard in behavioral health surveys in 2020. The second most frequently scored environmental standard was EC.02.03.05, the evidence that fire safety systems are maintained and inspected as required, the 10th most frequently scored standard.
EC Top 10 Most Frequently Scored:
 The authors also included a list of the ten most frequently scored EC elements of performance. For example, under EC.02.03.05, it is EP 15 which requires monthly inspection of fire extinguishers that was scored noncompliant on 15.5% of the behavioral health surveys in 2020. The ten most frequently scored EC elements of performance in 2020 were as follows:
The authors also included a list of the ten most frequently scored EC elements of performance. For example, under EC.02.03.05, it is EP 15 which requires monthly inspection of fire extinguishers that was scored noncompliant on 15.5% of the behavioral health surveys in 2020. The ten most frequently scored EC elements of performance in 2020 were as follows:
- 02.03.05, EP 15 Monthly fire extinguisher inspection – 15.5%
- 02.05.01, EP 9 Label utility system controls – 14.3%
- 02.05.07, EP 1 Monthly test of emergency lights – 12.8%
- 02.03.03, EP 1 Frequency of fire drills – 6.2%
- 02.05.07, EP 2 Annual test of battery power lights – 6%
- 02.06.01, EP 26 Furnishing and equip in good repair – 5.6%
- 01.01.01, EP 5 Written security plan – 5.4%
- 02.01.05, EP 7 Verify ITM for fire protection equipment – 5% (foster care)
- 02.01.05, EP 11 Verify smoke detectors sleeping rooms – 5% (foster care)
- 02.01.05, EP 13 Reassess safety during re-eval of case plan- 5% (foster care)
BH Top 10 Most Frequently Scored:
 We also noted an unintended, but interesting feature of this article, TJC included a listing of the entire top 10 most frequently scored standards, including clinical standards in behavioral healthcare settings for 2020. This is the first look we have seen of detailed scoring patterns from 2020.
We also noted an unintended, but interesting feature of this article, TJC included a listing of the entire top 10 most frequently scored standards, including clinical standards in behavioral healthcare settings for 2020. This is the first look we have seen of detailed scoring patterns from 2020.
The compilation you usually see twice a year for hospitals was not done due to limited survey volume in deemed programs. Behavioral healthcare as a non-deemed service was able to transition to remote surveys, thus there is more volume to report meaningful data. The top ten most frequently scored behavioral health standards were as follows, however the percentage of organizations where this was scored was not published, only the total number of organizations scored:
- 03.01.03 Treatment planning – 446
- 15.01.01 Suicide safety – 431
- 03.01.09 Assess outcomes of care – 366
- 02.01.11 Nutritional screening – 322
- 01.06.01 Staff competence – 284
- 02.02.05 Trauma, abuse, exploitation screening – 261
- 01.02.01 Verify staff qualifications – 257
- 02.01.09 Pain screening – 159
- 03.01.01 Medication storage – 155
- 02.03.05 Fire safety system testing – 152
 Portable Fire Extinguishers:
Portable Fire Extinguishers:
The last article we wanted to mention in EC News was the column titled: “What’s your question, What’s your solution.” This is where readers pose a question with the solution they developed. This month’s question dealt with the in-depth requirements for portable fire extinguishers and they provided a detailed summary of the many requirements for them. These include:
- No more than 75 feet of foot travel
- Appropriate signage if not clear that a fire extinguisher is physically present
- Installation on a hanger or in a cabinet
- Install at least 4 inches above the floor
- If 40 lbs or less, install no more than 5 feet above the floor
- If more than 40 lbs, install with the top no more than 3.5 feet off the floor
- In healthcare occupancy kitchens, a class K type extinguisher must be located within 30 feet of the broiler or other cooking appliance that uses fats
- In healthcare occupancy kitchens, a placard must be placed near each K type fire extinguisher warning that the kitchens fire protection system should be used before the K type fire extinguisher.
Personal Client Experiences:
We heard from two organizations who experienced a survey since TJC resumed survey activity and to a large extent the focus was similar to pre-pandemic. There were some other items noted, which may be a developing pattern, or may be idiosyncratic.
For example, in one case surveyors requested flip charts and individual inboxes and outboxes for materials requested. We also encourage organizations to use the inbox/outbox method for directing documents to the requesting surveyor, but we doubt that what was seen has become a new requirement from TJC.
Both organizations felt there was more CMS influence on this year’s survey, but that may be subjective. Both organizations mentioned targeted closed records requests including sedation records, discharge records to different settings, one pediatric cardiac surgery tracer, restraint records, critical results records, high risk for suicide records from inpatient and ED cases, and sedation records from different settings.
There appeared to be more requests for written policies that surveyors wanted to review than in the past and some testing of staff’s knowledge about surgical instruments and how to determine if they are safe to use.
In theory, the testing of staff knowledge about the appropriateness of when to use surgical instruments and when to reject them was something we had heard was going to start last year, but never got off the ground.
 We developed an Instrument Integrity Checklist for our consulting clients that we wanted to share this month with all readers. This tool can be used by quality or infection prevention department staff during their rounds or can be used by any area manager to test staff knowledge. Some organizations even post this checklist near where sterile instruments are stored to help remind staff how to inspect such supplies before use. Please take advantage of this free Patton proprietary tool and pass to all appropriate staff as you see fit.
We developed an Instrument Integrity Checklist for our consulting clients that we wanted to share this month with all readers. This tool can be used by quality or infection prevention department staff during their rounds or can be used by any area manager to test staff knowledge. Some organizations even post this checklist near where sterile instruments are stored to help remind staff how to inspect such supplies before use. Please take advantage of this free Patton proprietary tool and pass to all appropriate staff as you see fit.
Suitability for Survey:
 Last month we discussed the data CMS is analyzing relative to Covid-19 test positivity in counties throughout the United States, and both CMS and Joint Commission have been examining this to determine suitability for survey.
Last month we discussed the data CMS is analyzing relative to Covid-19 test positivity in counties throughout the United States, and both CMS and Joint Commission have been examining this to determine suitability for survey.
The interesting thing that we noted is the substantial increase in green counties and the corresponding drop in red and yellow counties. This speaks to the effectiveness of the vaccines, the physical distancing that we have all endured, and the hard work of the healthcare system and providers who should be proud of this achievement. Here is a comparison of the data we wrote about from 2/24 with this month’s data published on 3/23.
2/24/21 3/23/21 Change
Green 1327 Green 1892 +42%
Yellow 1541 Yellow 1154 -25%
Red 337 Red 113 -66%
So, the good news is things are improving. The bad news is things are improving and CMS has lifted its moratorium on surveys. CMS released QSO 21-16 on March 26 stating that complaint surveys, and revisit surveys can now resume.
In addition, follow-up requirements such as plans of correction for findings rendered in surveys that ended after January 20th, 2021 must be submitted within ten days following the release of this QSO memo.
Any hospital having difficulty allocating resources to develop and implement a POC because they are currently experiencing an outbreak of Covid-19, should contact their state agency or CMS location to request an extension.
Consultant Corner
Dear Readers,
Last month we mentioned that some exciting news would be coming this month. Well, drum roll please… WE HAVE A NEW WEBSITE!
We are thrilled to announce our newly designed, user-friendly website with you !
CAS clients, log in to our new and improved Patton Portal to check out your new available resources! This secure Portal gives you enhanced access to over 130 customizable survey prep and tracer tools. Please contact us should you need a reminder for your login and/or password.
Along with our CAS Tools, our Additional References (a NEW section of FREE tools, resources, and other useful files and links available for ALL readers) and our Patton Post Newsletters are all conveniently formatted and easily accessible for you to view or download. We have included either a description or table of contents for every entry so you can either sort by category or search by keyword to easily find what you are looking for.
We hope you find the website with its new functions easy to use and the new resources useful and informative! Have a wonderful month!
Jennifer Cowel, RN MHSA
JenCowel@PattonHC.com
Kurt Patton, MS RPh
Kurt@PattonHC.com
John Rosing, MHA
JohnRosing@PattonHC.com
Mary Cesare-Murphy, PhD
MCM@PattonHC.com
