December 2022
Inside This Issue
The December issue of Perspectives is not overwhelming with new requirements. In fact, we could say there is only one important simplification or reduction in requirements, and just one potential hardening of an existing requirement, plus the usual news and information updates.
Practitioner Privileging Cycle: 
The issue that may be simpler is that TJC is changing the privileging cycle for practitioners with privileges from no more than every two years, to no more than every three years. This has the potential to reduce the paperwork burden and time allocation processing re-applications, checking NPDB, and other information only every three years rather than every two years.
We would like readers to note that the language of this change permits you to make this change, but does not mandate you make this change by some deadline, or make the change at all. The reason we hesitate on this simplification is that it quite likely will require a medical staff bylaw change in many hospitals.
Currently MS.01.01.01, EP 14 requires the medical staff bylaws to include the process for privileging and re-privileging. Remember, the bylaws per MS.01.01.01 don’t require every little detail, but the basic process must be described in the bylaws, whereas associated details can be in other documents such as rules and regulations or policies.
The duration of privileges or the cycle is quite likely included in most medical staff bylaws and this is often a laborious document to modify. But it certainly is worth considering and starting the process to change as there may be significant time savings from making the change.
There are also timing implications to making this change which will require some planning. If you need to change your bylaws to implement the three-year cycle, you will need to plan out the timing of anticipated bylaws approval with the timing of distributing your reapplications. We should also point out that this change is not retroactive.
In other words, if your governing body approved an applicant for two years, this standard change does not affect that two-year period. Going forward, an organization can begin to authorize a three-year appointment, but for now, what is done is done.
Eye Wash Station Checks: 
The issue made more complex for some organizations is the frequency for checking eye wash stations. The March 2021 Consistent Interpretation column in Perspectives had stated that organizations could self-determine the frequency for eye wash station checks.
Additionally, an older FAQ had authorized organizations to conduct a risk assessment to determine a frequency for inspections. We recall encountering just a few organizations that went this route; most organizations stuck with a weekly test.
That earlier FAQ has disappeared from the TJC website and the current October 2021 FAQ references the most recent version of the ANSI standard Z358.1-2014. But what was not clear in the 2021 FAQ is that ANSI no longer permits this self-determined inspection cycle, they require a weekly inspection for all plumbed eye wash stations.
Joint Commission is now clarifying that omission of detail in its March 2021 Consistent Interpretation column and October 2021 FAQ. So, if you were one of the rare organizations using an inspection cycle other than weekly for eye wash stations based on a risk assessment, you will need to update your process to now include weekly inspections.
Health Care Equity: 
The news and information pieces in this month’s Perspectives are much easier to digest. TJC has created a health care equity resource center that may prove useful to organizations implementing the new leadership standards on this issue. We noted that the new site has links to resources for each of the new elements of performance plus various clinical topics.
Thus, if you have already planned out your efforts on most but not all EPs, you can use this site to gather some ideas on how you might go about implementing that particular EP. If you are already far along in your process you can check the site to obtain and compare the suggestions with what you have planned and perhaps add to your initiative.
TJC also noticed that their new electronic and print manuals mistakenly did not include the new health care equity standards in the LTAC, psychiatric, surgical specialty or swing bed services applicability grids for these specialty hospitals for its hospital and critical access hospital programs.
However, the requirements under LD.04.03.08, EPs 1-6 do apply. So, chalk up one RFI to the accreditor for now, and if you are one of these special hospital types that concluded applicability was being delayed, you will have to make your case to the surveyors if you are behind in installing these new requirements.
LIP Terminology Removed: 
The last bit of news in Perspectives is that TJC is eliminating the term licensed independent practitioner, or LIP. The term LIP was for many years unique to TJC, and to many physicians it was a source of derision at TJC for calling them LIPs. So that term has been cast off and is now replaced by the term practitioner/provider/physician.
TJC states that the new glossary will have updated definitions for staff, clinical staff, physician, practitioner, and provider. While we can see the 2023 standards in E-Edition, we are not sure the new definitions have been added to the glossary.
As we are writing this newsletter, we noted that the new term provider is not yet in the glossary and LIP is still referenced. Take another look at the glossary as we get closer to 2023 to identify these definitions and determine if you need or want to change any bylaw or policy references.
Health Information Privacy and Security:
This month’s Consistent Interpretation column is somewhat mundane discussing IM.02.01.01 and IM 02.01.03 which establish requirements for health information privacy and security. These two standards are not heavy hitters, being scored respectively in only 2.2% and 0.6% of hospital surveys last year.
As usual, the guidance/interpretation section helps to parse where surveyors should place their findings. The surveyor observations sections do provide insight on issues that might be scored and provides examples of issues surveyors have made note of and their techniques.
The two issues we see most commonly are paper documents left at the nursing station which are visible to visitors in the vicinity of the countertop and electronic data left active on the screen of a hallway or office computer. Screen savers which activate quickly and automated logoffs can help, as well as training staff to log out when leaving their terminal.
Water Management: 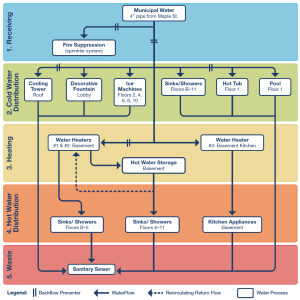
This month’s EC News has a good article about the Joint Commission’s water management standard, EC.02.05.02. The authors report that TJC gets many questions about this standard and we know that some of our clients struggle to understand the requirements under this standard.
TJC reports that one of the most frequently asked questions is about the level of detail required for the basic water management diagram (EP 2). They advise creation of a simple diagram such as the one published in the CDC toolkit on legionella.
You will note that this diagram does not identify every sink, shower, or toilet, but rather identifies that their water system distributes to sinks, showers, and toilets on certain floors, as well as other water features in the cold and hot water distribution green and orange sections.
One question we have received from a client is about any mandatory flushing/running of showers in closed sections of the organization. Unfortunately, there is no absolute guidance on this issue, but rather the guidance should come from your internal risk assessment.
This risk assessment should consider all of the factors that tend to contribute to the safety of your water system such as disinfectant levels, temperatures of the water and, if you are testing water quality, the results of that testing. Bottom line, yes flushing or running water systems in closed areas is expected, but the frequency may vary based on your risk assessment.
In addition, your risk assessment should consider the patient populations served, which may include immunocompromised patients who are more vulnerable to potential water borne/aerosolized infections.
This particular standard, EC.02.05.02 has not yet made the top ten, but it is one that should be focused on. The limited scoring at this time may be related to the newness, rather than any perception that compliance with this standard is easy.
We would certainly suggest distributing the article to your facilities leadership for their use.
Business Occupancies Requirements: 
EC News has another article on new requirements for 2023 that focuses on the changes made just a few months ago to the LS.05 standards which affect business occupancies. We wrote about these in our October Newsletter. These will likely be challenging as business occupancies have not undergone a lot of scrutiny from an LS/EC perspective in prior years.
This month’s EC News reproduces the new standards and we would certainly suggest conducting a self-evaluation and gap analysis in your ambulatory facilities that are identified as business occupancies. It may take time for scoring of these new standards to ramp up, but it also may take time for healthcare organizations to become compliant. Thus, the self-evaluation and gap analysis might be protective on future surveys.
FGI Guideline: 
Lastly, there is an important article in EC News that advises readers that effective January 2023, The Joint Commission is referencing the most current design and construction guidance from the Facilities Guideline Institute, or FGI. This will be the 2022 FGI Guideline – your 2022 TJC accreditation manual references the 2018 FGI guideline.
This is of course important if you are planning new construction, but it is also important if you are planning any renovations, which would require the renovation to be brought up to the most recent standards. This seemingly simple modification to the TJC standards has major implications as the FGI document is an entire book of guidance, not merely a single standard.
Readers will want to verify they have access to the 2022 FGI subscription and are using its standards when preparing for new construction or renovation. The EC News article discusses specific changes in the FGI guidance for emergency departments, burn trauma ICU beds, telemedicine settings, the number of airborne isolation rooms a hospital should have, and lactation rooms.
If you have any renovation or construction plans for these areas you will want to be sure to read the latest FGI guidance.
LTC Covid-19 Therapeutics: 
CMS has one new QSO memo, QSO-23-03 issued November 22, 2022 targeted to all provider types, but with particular emphasis on the long-term care industry. The memo discusses the importance of timely treatment with Covid-19 therapeutics including monoclonal antibodies and oral antiviral agents for high-risk individuals.
After reading this CMS memo, we did find multiple internet references discussing the limited utilization of federally purchased Covid therapeutics. This memo clearly seems to target and promote greater utilization of these agents. The CMS memo has two sentences that seems of significant importance to readers.
On page 1 of this memo CMS says, “Every patient who tests positive for Covid-19 should be evaluated to determine whether the use of an available therapeutic is appropriate.” On page 2 they state, “CMS reminds providers and suppliers that, even when a patient’s symptoms may not initially present as severe, individual risk levels should be considered when deciding whether or not to prescribe an appropriate course of treatment.”
Looking at guidance like that from a compliance perspective has the potential to create questions about medical record documentation. The absence of prescribing a Covid therapeutic may mean it was considered and ruled out as not appropriate. However, the absence of prescribing is not the same as a progress note stating the evaluation of appropriateness. For example, “the patient does not have comorbidities or meet the CDC definition of high risk, therefore a Covid therapeutic was not prescribed.”
We may be over reading this simple two-page memo, but you may reach similar conclusions. We suggest a careful read of this memo to determine your course of action.
Sampling and SOPs: 
We did have a chance to review the recently released update to USP Chapter 797 we briefly discussed last month. Obtaining a copy is the first step in the process and you will want to do this as soon as possible to start your analysis. It is available as an online version only from USP.org within their Compounding Compendium; they are not selling PDF or paper copies.
Reading, studying, and understanding how this version compares to earlier version online is very tedious, but if you do it the old-fashioned way and print a hard copy and then use the print/save as function to save the document as a PDF, it is far less challenging.
During our initial read, two issues jumped out at us, the first is additional sampling we mentioned last month and the second was the number of references to SOPs, or standard operating procedures, required.
Essentially you should think of each section in the reference document as requiring its own SOPs, or several, for that section. This is not a complete listing, but we noticed requirements for SOPs on aseptic techniques required for immediate use preparations, training and competency, obtaining, conducting and incubating microbiologic sampling, hand hygiene, garbing, cleaning and disinfection, calibration of automated compounding devices, terminal sterilization and depyrogenation if performed, visual inspection of compounding products, labeling, and management of complaints about prepared products.
In addition, these SOPs will need to be reviewed and modified as needed every 12 months. The presence or absence of a required SOP is an easy and objective finding for an accreditation surveyor. Over time surveyors will likely develop skills at evaluating the quality of the SOP and of course the actual observation of implementation of the SOP by staff. But for now, failure to have a required SOP is an easy find.
New Acronyms and Terminology Changes: 
There is also new terminology and acronyms that staff will need to become familiar with. The previous low risk, medium risk, and high-risk compounding definitions have been replaced by what they call category 1, category 2, and category 3 sterile compounds. These categories are to a large extent defined by the compounding area conditions and how long a beyond use date you need to assign to the compounded product.
A new acronym has been described called RABS (restricted access barrier system). This is an enclosure providing ISO 5 clean air and there are two main types of enclosures with two previously known acronyms, CAI (compounding aseptic isolator) and CACI (compounding aseptic containment isolator) for hazardous compounding.
One very important change here is that the CAI must be placed inside an ISO 7 environment except when used to prepare a short BUD category 1 sterile compound. Today many of these CAIs are found in a segregated compounding area using today’s longer USP beyond use dates. This change may identify renovation needs or potential workload issues if shorter dating is all that can be used.
The new Chapter 797 has multiple references to USP Chapter 800 for hazardous compounding. USP Chapter 800 remains as previously published, but its requirements have not been in effect, so far. However, when the new USP Chapter 797 becomes official in November 2023, that date also becomes the official implementation requirement date for Chapter 800.
Next Steps: 
There are 12 months to go before these new requirements take effect. Accreditors and state board of pharmacy surveyors will be learning about the new chapters during the year leading up to next November.
Your pharmacy staff have a much more rigorous task as they will need to expedite the learning process so that they can change existing practices, develop the previously mentioned SOP’s, update training materials, conduct training and competency and either find a vendor to perform and incubate the monthly surface sampling or gear up to take on that responsibility as a staff function.
The first step is of course purchasing the new Chapter to begin the analysis. We anticipate that USP, State, and National pharmacy associations will all be developing in person and online training to help understand the new requirements. You will want to get this initial access and training conducted ASAP so that you can identify time dependent road blocks and work around them prior to November 2023.
FAQs and the One Hour Rule: 
In addition to releasing the new Chapter 797, USP also published almost 40 pages of FAQs, totaling 195 questions, adding more details about interpretation of the chapter. We wanted to bring two of these FAQs to your attention. These are # 20 and #21.
FAQ #20 defines that simple functions, such as removing a dose from a sterile container without further manipulation, is not compounding and thus is not covered by USP Chapter 797. This declaration of applicability then leads to FAQ #21 which states that spiking an IV bag is also not compounding, thus any limitations that might currently exist such as the “One Hour Rule,” are not supported by rationale from USP Chapter 797.
We noticed that TJC has now removed their FAQ addressing the “One Hour Rule” on pre-spiking IV bags. If your organization managed to eliminate this practice, we would encourage readers to not restart pre-spiking, but if you had previously been unable to eliminate the process, we offer the following advice: We recommend reviewing the FDA package inserts for IV solutions and administration sets to verify there is no MIFU that prohibits pre-spiking.
We did notice one IV solution manufacturer that advised removal of the outer plastic wrap “immediately prior to use,” which would mean pre-spiking is not an option for their products. In addition, we would also suggest developing a policy if you are going to authorize the process of pre-spiking.
In development of your policy, we would also suggest using an interdisciplinary risk assessment, detailing that you have concluded the action is safe to perform and necessary due to time constraints.
Consultant Corner
Dear Readers,
We are thrilled to introduce Julia Finken, BSN, MBA, CPHQ, LCSSMBB, Senior Vice President of Accreditation & Regulatory Compliance!
Julia joined the team in November and comes with a remarkable 25+ years of healthcare industry expertise with a 17+ year tenure with The Joint Commission. At TJC, she served as a surveyor, the Associate Director of Business Development, and most recently as the Executive Director of Business Development for the Behavioral Health Care and Psychiatric Hospital Programs.
Her exemplary career has been dedicated to developing and implementing health care programs to balance efficiency, quality and financial outcomes in order to improve daily operations, quality of care, financial performance, business and marketing strategies, and so much more for a successful health care operation.
The expertise and integrity she exudes will prove to be an unmatched asset to you and your organization in helping you achieve compliance and accreditation and accomplishing patient safety. Please join us in giving her a warm welcome!
We wish you a very Happy Holiday Season and Happy New Year. May it bring you joy, new adventures, and good fortune.
Jennifer Cowel, RN MHSA
JenCowel@PattonHC.com
Kurt Patton, MS RPh
Kurt@PattonHC.com
John Rosing, MHA
JohnRosing@PattonHC.com
Mary Cesare-Murphy, PhD
MCM@PattonHC.com
