June 2021
Inside This Issue
Inpatient Insulin Pumps and Glucometers:
There are no new standards or changed requirements discussed in this month’s issue of Perspectives. There is one article we would consider a “must read” and that is this month’s version of the Consistent Interpretation column. This edition is ten pages long and discusses inpatient requirements for patients admitted who use their own insulin pump and or blood glucose monitoring device.
These devices are becoming more and more common for patients to use, and hospitals to identify during admission assessments. The usual format for this column identifies a standard and EP, followed by surveyor observations and guidance from the standards interpretation group on where to score or not score the particular observation. The nuances of where to score is of lesser importance to many readers, but the question about if something is a requirement or not, and what is that requirement is often very important to know and understand.
You will want to do a very careful read of the entire document, and preferably have it read by a team of individuals to ensure that you identify any potential gaps in your policies and processes. We had to read through it several times to try and pick out the articulated requirements which may not have been clear previously.

The first standard and EP discussed is HR.01.05.03, EP1 which requires ongoing staff education and training. The yellow shaded “Noncompliance Implications” section contains four clear requirements that not every organization may have associated with personal insulin devices. These are:
- If patients are permitted to self-manage their own devices (pumps) there must be a written process to guide safe and accurate self-administration. They reference MM.06.01.03, EPs 1, 3 and 7.
- If patients are permitted to self-manage their own devices (pumps) then organizations also need to have a process in place, such as a learning needs assessment, that considers clinical or environmental factors that may be barriers to self-administration. E.g., a patient who may be receiving narcotics that may impair cognition.
- If patients are permitted to self-manage their own devices (pumps) there must be policies and procedures to guide nursing care. They reference NR.02.02.01, EP 1.
- If patients are permitted to self-manage their own devices (pumps) then staff must be provided education and training regarding the organizations policies and procedures for pump use. TJC also makes it clear that the training does NOT need to teach staff how to operate each insulin pump. TJC does, however, mention that such training may need to include additional assessment activities or safety procedures when caring for pump patients.
The discussion of standard LD.04.01.07, EP 1 uncovers another requirement when it guides surveyors to review an organization’s policy for verification of device accuracy and to score it if the organization is not adherent to that policy. Thus, our conclusion is:
- If patients are permitted to self-manage their own devices (pumps) there must be a process to verify the accuracy of the pump. This is further explained later in their discussion of PC.02.01.03, EP 1. Here TJC provides an example of how you might verify device accuracy by performing a blood glucose test with the hospital glucose meter and comparing the results with the patients’ device reading.
The discussion of MM.03.01.05, EP 1 (the outside the hospital or brought from home medication standard) provides additional depth of information about what should be contained in your policies and procedures for pump use when it states:
- The organization’s defined process should outline requirements, such as assessment and care of the site, documentation, monitoring, and response should the device malfunction.
The discussion of MM.04.01.01, EP 1 (the medication orders standard) identifies that the hospital, if they allow the use of a patient’s personal insulin pump:
- The hospital must have a process for ordering the insulin.
The discussion of MM.04.01.01, EP 2 then adds depth to the previous requirement when it states that if the organization allows the use of a patient’s personal insulin pump:
- The pump settings need to be verified so the provider (i.e., prescriber) is aware of the basal and bolus dosages being administered.
The discussion of MM.06.01.03, EP 3 (the medication self-administration standard) details a requirement which we believe most readers already see in the element of performance which requires:
- There must be education of patients and families on self-administration of medication, and this will include insulin pumps.
Similarly, the discussion of MM.06.01.03, EP 7 describes the current expectation to verify the competence of patients or families to self-administer medications.
- The hospital determines that the patient or family member who administers medication is competent in medication administration before allowing them to administer, and this includes insulin pumps.
The discussion of PC.02.01.03, EP 7 is interesting. The EP requires the hospital to provide care, treatment and services using the most recent patient order. We would not have foreseen how this EP corresponds the following requirement, but apparently TJC sees the link.
- When a continuous glucose monitoring device is used, the process must comply with the device manufacturer’s instructions for use (MIFU) for calibration to ensure that the device provides accurate results.
We have discussed MIFU many times the last few years and the struggle hospitals have to ensure that staff have access to the MIFU and use the MIFU associated with the appropriate device. This may be complex for personal blood glucose/insulin devices in that patients may be admitted wearing different brand devices. We would encourage our readers to have someone pull and analyze the MIFU for the major brands of devices you see in your organization to identify if there are any unique quality control or other procedures that must be performed for specific brands. If you identify unique requirements you would want to build those into your policies and procedures for device use.
RC.02.01.01, EP 2 describes medical record documentation requirements and one of the many bullet points is the requirement to document medications administered including the strength, dose, route, date and time of administration. The guidance provided is somewhat nebulous in that it states:
- “The organization determines how to document in the medical record the dose of insulin administered via a self-administration device.” However, when we look back at the EP language, we would conclude that the documentation process you develop should also include the medication name, dose, route, date, and time of administration.
Lastly there is one standard discussed, IM.02.02.01, EP 3 (the prohibited abbreviation standard) that does not seem to fit with the rest of this discussion, but it is illuminating for a different reason. Insulin products are referenced as U-100, or U-500 to denote the concentration of the insulin and this use of the abbreviation U is not prohibited. It is not prohibited because it is part of the name of the insulin product, it is not an amount prescribed to administer to the patient. Thus, prescribing U-100 insulin would be acceptable, but prescribing five U of it would not be acceptable.
Insulin Pump/Continuous Glucose Monitor Policy:
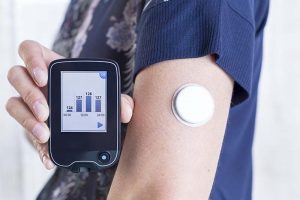
This lengthy article from TJC and our discussion is likely to cause many of our readers to want to review and edit, or develop a hospital policy for use of these devices. A quick internet search identified several resources that may prove helpful to this effort. The first one identified was a 2020 policy from the Yale New Haven Health System. This is nice because if might provide you a template of ideas on how to start your own policy.
They provide some useful guidance on educating the patient that the hospital may need to remove the device or discontinue its use and to advise the patient that the hospital may not stock all the unique vendors supplies and that they should be sure to bring adequate supplies to the hospital for scheduled or needed site changes. They also provide detailed ordering instructions and requirements.
There is advice about not allowing the patient to wear the insulin device into a radiology procedure room where there is concern about the device being damaged by radiation or incompatibility with MRI equipment.
Another valuable resource comes from The American Society of Health System Pharmacists Advantage service has a sample insulin pump policy, which is posted to their website.
A third resource comes from The Institute for Safe Medication Practices, ISMP, publicly available document entitled, “Safe Management of Patients with an External Subcutaneous Insulin Pump During Hospitalization” that includes 48 specific recommendations for assessment, documentation, ordering, and monitoring of patients using an insulin pump.
We found a fourth article entitled, “Continuous Glucose Monitors and Automated Insulin Dosing Systems in the Hospital Consensus Guideline,” published in the Journal of Diabetes, Science and Technology Vol 14(6) 2020. In this guideline a panel of experts developed consensus around 78 specific recommendations for clinical practice and research relative to the of CGM and AIDs.
TJC FAQ on Vaccinated Staff and Masks:
 During the past month, TJC posted a new FAQ, “COVID—Vaccinated Staff Members and Masks” in the infection control chapter on the question of: Can healthcare staff who have been vaccinated stop wearing masks for source control? The FAQ answer provides links to three CDC documents and one lengthy document from OSHA.
During the past month, TJC posted a new FAQ, “COVID—Vaccinated Staff Members and Masks” in the infection control chapter on the question of: Can healthcare staff who have been vaccinated stop wearing masks for source control? The FAQ answer provides links to three CDC documents and one lengthy document from OSHA.
Basically, it says that healthcare settings and healthcare staff are different from the general public and staff should not infer those flexibilities recommended for the general public apply to healthcare settings. We would encourage our readers to review carefully the CDC Guidance for the Healthcare industry. This document has a lot of information for our industry about visitation procedures, screening of visitors to the hospital, and mask wearing.
The OSHA document, is both lengthy, comforting, and concerning in that it describes an inspection initiative in healthcare settings to ensure that procedures remain in place to protect workers. While protecting healthcare workers certainly sounds great, the visit from an inspection agency can at times feel very punishing to the very staff who they are trying to protect.
TJC FAQ on Medication Autoverification:

There was a recommendation to the American Society of Health System Pharmacist’s House of Delegates in 2020 for regulatory authorities to clarify their stance on what is called “autoverification” of medication orders.
An ASHP survey of hospitals in 2019 reported that 62% of hospitals used autoverification for some types of medications. Using autoverification, a new medication order would go from the prescriber to reviewed/approved status without a pharmacist’s review, relying instead on artificial intelligence-based verification software.
The new FAQ from Joint Commission, “Medication Dispensing – Use of Auto-verification Technology” responds to this request from ASHP and makes it clear that the autoverification process is inconsistent with the Joint Commission’s standards which do require a pharmacist to review each new medication order, except in a very few limited circumstances such as an emergent or urgent clinical situation or when the provider is in attendance with the patient and in control of the medication.
Hazardous Chemicals and Waste Hauling:
 The lead article in this month’s EC News is a review article on hazardous chemicals. This is a good overview of requirements from TJC in EC.02.02.01, OSHA, EPA, and even the Department of Transportation. There is a less well-known requirement from DOT to have the person who will sign the hazardous waste manifest for your waste hauler to be trained at least every three years on the Federal requirements for waste hauling.
The lead article in this month’s EC News is a review article on hazardous chemicals. This is a good overview of requirements from TJC in EC.02.02.01, OSHA, EPA, and even the Department of Transportation. There is a less well-known requirement from DOT to have the person who will sign the hazardous waste manifest for your waste hauler to be trained at least every three years on the Federal requirements for waste hauling.
Fortunately, DOT has an online training program you can use, but we still see this issue scored with some frequency on TJC surveys. The article is definitely worth sharing with your facilities team and in particular it should be shared with the individual with primary responsibility for maintaining and evaluating your hazardous materials plan. The authors provide many recommendations for compliance and some could perhaps be useful additions to your hazmat plan and process.

Keeping Facilities Safe for Seniors:
There is also an interesting article on designing healthcare facilities for a growing senior population. If you are doing any renovations or new construction this has some interesting suggestions for making the physical environment safer for seniors.
2020 Most Frequent EC/LS & Ambulatory Standards:

Lastly, there is an article detailing the 2020 most frequently scored EC and LS standards and most frequently scored standards for all chapters in organizations accredited using the ambulatory healthcare facilities manual. The issues scored in ambulatory facilities will not be a surprise to our readers as they correspond to many of the usual suspects in hospital surveys.
For example, the most frequently scored standard is IC.02.01.01, general infection prevention methods, and the second most frequently scored issue is IC.02.02.01, usually related to high level disinfection and sterilization. TJC also provided their EP analysis for the highest risk rating on the SAFER Matrix and as you might expect the issues of high-level disinfection and sterilization were the highest risk rated.
The most frequently scored EC chapter findings were as follows:
EC.02.03.05 Maintaining and inspecting fire safety features – 41%
EC.02.05.01 Managing utility system risks – 41%
EC.02.05.07 Emergency power – 40%
EC.02.02.01 Hazardous materials and waste (see reference above) – 36%
EC.02.04.03 Medical equipment ITM – 30%
EC.02.03.03 Fire drills – 24%
EC.02.05.05 Utility system ITM – 20%
EC.02.06.01 Safe, sanitary environment catch all standard – 20%
EC.02.06.01 Manage fire risks – 18%
EC.02.05.09 Medical gas/vacuum ITM – 7%
TJC also supplied the most frequently scored standards in the life safety code chapter and these were as follows. However, to change things up, TJC reported on only the six most frequently scored LSC chapter standards.
LS.03.01.35 Maintain firefighting equipment – 43%
LS.03.01.10 Fire protection features, (barriers, doors, sprinklers) – 39%
LS.03.01.30 Protect from fire and smoke – 25%
LS.03.01.34 Maintain fire alarm systems – 14%
LS.01.01.01 Comply with life safety code, maintain BBI – 14%
LS.03.01.20 Maintain the means of egress – 13%
COVID-19 Data:
The last four months we discussed the data CMS is analyzing relative to Covid-19 test positivity in counties throughout the US. CMS and Joint Commission have been examining this data to determine suitability for survey.
The good news is the test positivity appears to be going down with more counties identified as green and fewer in red or yellow. However, as we mentioned earlier in our discussion of new FAQs, things may be getting back to normal in our communities, but protective measures still need to be in place in healthcare settings.
2/24/21 3/23/21 4/27/21 5/28/21
Green 1327 Green 1892 Green 1795 Green 2286
Yellow 1541 Yellow 1154 Yellow 1209 Yellow 826
Red 337 Red 113 Red 204 Red 44
Nursing Homes and Covid-19 Vaccinations:
On May 11, 2021 CMS issued QSO-21-19 directed to the nursing home industry mandating education of nursing home residents and staff about the value of Covid-19 vaccination. This does require medical record documentation of effort to educate the resident or patient’s representative about the vaccine.
Documentation of effort to educate staff and offer vaccination is also required, although how this must be documented is not stipulated. There are two new F tags for scoring any noncompliance identified on the education/offering, as well as the requirement to report vaccinations administered.
Hospitals have had a significant reporting requirement to HHS on capacity and utilization data since 2020 using a much larger data set. We noted that the FAQ HHS developed on this requirement has been updated as of May 27, 2021 expanding the number of data elements from 32 to now 47. They have also identified an implementation date for the revised requirements of June 10, 2021.
PPP: Hazardous Waste Medications:
 In 2019, we reported on the EPA’s revised regulations relative to hazardous medication waste. That year we also discussed preparation healthcare organizations were making to implement USP Chapter 800.
In 2019, we reported on the EPA’s revised regulations relative to hazardous medication waste. That year we also discussed preparation healthcare organizations were making to implement USP Chapter 800.
The requirements of USP Chapter 800 have been delayed while the authorizing chapter USP 797 is being revised, but NIOSH still has requirements for worker protection from hazardous medication.
With the EPA having requirements for handling hazardous pharmaceutical waste and NIOSH/USP having different requirements for protecting staff from hazardous medications, the discussion can get confusing.
We wanted to bring to your attention a very useful article we saw in the May 2021 issue of Pharmaceutical Purchasing and Products, available free of charge, “Demystify New Regulations for Hazardous Waste.”
This article does a nice job of identifying the similarities and differences between these different areas of focus. The authors included a Venn diagram depicting which categories of medication are the focus of NIOSH, which categories are the focus of the EPA and which medications in the middle are the focus from both guiding directives.
We would encourage our readers to download this article as there may be opportunities to refine your waste handling processes.
Consultant Corner
Dear Readers,
No super exciting or imperative news coming from us here at Patton this month.
We hope that you all have a wonderful month and enjoy the start to summer!
Be safe and be well!
Jennifer Cowel, RN MHSA
JenCowel@PattonHC.com
Kurt Patton, MS RPh
Kurt@PattonHC.com
John Rosing, MHA
JohnRosing@PattonHC.com
Mary Cesare-Murphy, PhD
MCM@PattonHC.com
