June 2023
Inside This Issue
 ORYX Data Submission:
ORYX Data Submission:
The June issue of Perspectives is pretty thin with only one approaching deadline for action related to submission of ORYX data coming on June 14th. This relates to the completion of and availability of The Joint Commission’s direct data submission platform.
Critical Access Hospital Standards:
 There is also an announcement for critical access hospitals of some standards changes that are the result of revised CMS guidance for CAHs. The new requirements appear very similar to existing requirements already in the acute hospital standards. The most notable areas subject to these changes include restraint and seclusion and CAHs that are part of multihospital systems who might choose to use a combined governing body and or medical staff. More to follow later in this section related to new interpretations of the combined and non-combined medical staff requirements.
There is also an announcement for critical access hospitals of some standards changes that are the result of revised CMS guidance for CAHs. The new requirements appear very similar to existing requirements already in the acute hospital standards. The most notable areas subject to these changes include restraint and seclusion and CAHs that are part of multihospital systems who might choose to use a combined governing body and or medical staff. More to follow later in this section related to new interpretations of the combined and non-combined medical staff requirements.
Integrity of the Egress: 
This month’s Consistent Interpretation column is on LS.02.01.20, EP 1 regarding maintaining the integrity of the means of egress. This EP deals with the prohibition on supplemental locks requiring tools, keys, or manipulation of supplemental locking mechanisms on egress doors. Somewhat surprisingly this was scored noncompliant in almost 20% of hospital surveys last year.
The guidance/interpretation section provides insight into detailed NFPA requirements for these doors and locking mechanisms that are required, but not a written part of the standard or its elements of performance. We would suggest readers send this section to facilities leadership along with the question about your hospital’s compliance with these requirements for all egress doors.
Sometimes we see organizations that are concerned about security of the building and want to prevent people from getting in certain doors, but usually those doors can’t delay people already in the hospital from getting out. There are of course certain exceptions for controlled areas such as inpatient psychiatry, and labor and delivery, along with detailed requirements to make egress easy for staff in an emergency.
 Preventing Light Source Burns:
Preventing Light Source Burns:
Perspectives also has a brief article summarizing their Quick Safety #69 published back in April on preventing light source related burns from laparoscopy, thoracoscopy and arthroscopy. Perspectives does duplicate the suggested safety actions; however, the original Quick Safety has more background information describing the nature of the problem and references. The suggested safety actions are worth evaluating with your operating room leadership. The link provided in the Perspectives article did not work for us, but we were able to find the publication using their search tool along with some trial and error.
 Single Medical Staff Option Leading to New Burdens:
Single Medical Staff Option Leading to New Burdens:
As part of its burden reduction efforts back in 2016 – 2019 CMS developed new regulations that would permit hospital systems to have a single governing body and medical staff if they wanted. This was optional and hospitals could choose one without the other. TJC responded with matching standard and elements of performance.
Relative to medical staff the enabling standard was MS.01.01.01, EP 37 which authorized the formation of a single medical staff across multiple hospitals in a system providing certain requirements were met. Specifically, EP 37 required the medical staff bylaws in a multihospital system that had created an integrated medical staff to include a statement that medical staff members had a right to opt out of the integrated medical staff by a vote of the majority of medical staff members.
Our interpretation of this was that it created an if/then requirement, meaning if they had an integrated medical staff across multiple hospitals in the system, then those hospital’s medical staff bylaws had to include this guidance on opting out. Since EP 37 was created we had not seen any RFIs describing noncompliance.
Recently things changed when we saw three survey reports with the same finding related to MS.01.01.01, EP 37 that surprised us. Each of these hospitals were part of systems but had not decided to have an integrated medical staff for the system, but rather still had individual hospital medical staffs. These three hospitals were cited by TJC for not including language in their medical staff bylaws informing members of their right to opt out of an integrated medical staff, even though they did not have an integrated medical staff. Since we had not seen RFIs like this previously we were sure it was incorrect, however TJC confirmed the findings were appropriate.
We re-reviewed the CMS State Operations Manual for Hospitals and found the following language under tag A-0349, 482.22(b):
“A hospital that is part of a hospital system is expected to have medical staff bylaws, rules and requirements that address the regulatory requirements of 482.22(b)(4)(i)-(iv) related to using a unified medical staff, including processes under the bylaws for voting to accept or opt out of a unified medical staff. This is the case even if the hospital currently does not use a unified medical staff.”
Thus, the if/then logic presumed from reading the language used in EP 37 appears to be incorrect. Hospitals that are part of a system, but who have had no interest in creating a unified medical staff may need to add content to their medical staff bylaws describing how the medical staff can choose to opt out of the integrated medical staff in the system.
In other words, the burden reduction initiative previously welcomed may actually lead to new burdens including a new round of edits to the medical staff bylaws.
 BHC Workplace Violence Standards:
BHC Workplace Violence Standards:
The lead article in EC News discusses the proposed workplace violence prevention standards for behavioral health care and human services organizations (BHC) currently undergoing a field review. These draft standards appear to closely correspond to the standards previously developed for the hospital program. As workplace violence is such an important issue for the behavioral health field, readers may wish to complete the field review questions and offer their own suggestions for enhancements.
 Emergency Management Drills and HVA Development:
Emergency Management Drills and HVA Development:
This month’s EC News has three articles on emergency management that will be of interest to your EM committee. The first two articles discuss making drills more effective, the different types of drills available to use, their features and estimated planning time. The second EM article is particularly helpful in that it provides 10 suggestions for making EM exercises more effective through careful planning, design, and evaluation of the exercise.
The third EM article describes steps in the creation of your hazard vulnerability assessment. Perhaps more importantly the author suggests involving your community partners in the development of the HVA so that they may provide their perspective on community preparation and their ability to help support the organization should the likely hazards occur. That may lead to better planning and design of drills for likely hazards where mitigation and recovery is more questionable.
These three articles should be shared with and analyzed by your EM committees. You may want to consider a longer-range drill plan, expansion of your internal planning team, inclusion of more community partners and modification of the types of drills and scenarios you were considering.

Risk Assessments:
The last article discusses the concept of risk assessment and provides some examples of different types of risk assessments which are required to be performed, particularly for NFPA requirements. We believe the concept of risk assessment to be very helpful, not always well understood, and very often the term risk assessment is overused when applied to many different processes, functions, and purposes.
The term used earlier in EC News on hazard vulnerability assessments is itself a risk assessment, but perhaps having its own unique nomenclature makes it easier to understand. Nevertheless, this EC News article does provide guidance on the OR humidity risk assessment many organizations fail to perform when the accept the 20% humidity threshold in operating rooms. They also provide helpful guidance on the risk assessment required to determine the inspection, testing, and maintenance interval for medical gas systems.
Infection Prevention Standards Status:
We had mentioned last month that there would be a field review planned for new infection prevention standards being developed. As we were drafting this newsletter, we see that the link has just become active on the prepublications section of The Joint Commission website for both the proposed new standards and the field review on those draft standards. This is an important opportunity to help shape the process, so we suggest taking a look, and providing feedback as appropriate.
 Implementation Deadline Approaching:
Implementation Deadline Approaching:
This being June means we are five (5) months away from the implementation deadline for the new USP Chapter 795, 797, and 800. A lot of focus has been placed on Chapters 797 and 800 for several years as drafts were being developed and modified. Hopefully your organization is well along in its planning for 797 and 800.
At this time TJC has indicated that it will survey the requirements of Chapter 795, which governs nonsterile compounding activities, in its home care accreditation program only. Nonsterile compounding has been part of a pharmacist’s academic preparation for perhaps several thousand years, but to a large extent nonsterile compounding has become a smaller part of actual pharmacy practice for many pharmacists during the last 50 years. However, as an official USP publication you will want to check on the applicability of Chapter 795 as a result of State Board of Pharmacy regulations.
In many healthcare facilities nonsterile compounding takes place wherever you can find clear space near a sink. As we reviewed the new requirements here are some highlights that readers should be preparing for by November. Some will require the development of unique processes, while others may be able to utilize existing process within the healthcare organization such as complaint management and employee health.
Designated Person: The chapter requires each organization to appoint one or more individuals to oversight of the entire process.
Training and Competency: The chapter will require training and competency verification in readiness for November and every 12 months thereafter on expected hand hygiene, garbing, cleaning, and sanitizing, handling and transporting components and compounded products, measuring and mixing, proper use of equipment used, documentation of master formulation records and records of compounding.
Personal Hygiene: A process to identify and report rashes, recent tattoos, oozing sores, conjunctivitis, or active respiratory infection to the designated person will need to be designed. It is likely healthcare organizations already have an employee health reporting process that can be adopted.
Attire Policy and Procedure: Outer garments, jewelry, ear buds and headphones will all need to be prohibited in the nonsterile compounding space.
Garb and Glove Requirements: Gloves are required for all nonsterile compounding and policies should be developed for other garbing requirements such as shoe covers, head or hair covers, and retaining gowns in the compounding space if they are going to be reused.
Compounding Space: A designated space for nonsterile compounding must be identified and other activities not performed simultaneously if it is shared space.
Temperature Monitoring: The storage space must be monitored at least daily to ensure the space is appropriate to the products stored.
Cleaning and Sanitizing: The compounding devices, work surfaces, floors, walls, ceilings, and storage shelving will need SOPs for cleaning and records documenting that this is performed at the frequency required by the chapter.
Master Formulation Records: Detailed records (a recipe) describing the procedures on how to compound your products must be developed.
Compounding Records: Detailed records must be maintained for all compounds prepared with a minimum of 12 data elements required by the chapter.
Beyond Use Dates: Must be established for compounded products based on official compounding monographs or Table 4 in the chapter.
SOPs: Standard operating procedures will need to be developed for all activities in nonsterile compounding.
QA and QC: There must be a QA/QC process for adherence to procedures, detection of problems, evaluation of complaints and corrective actions.
 CMS and Accrediting Bodies Compared:
CMS and Accrediting Bodies Compared:
During the past month CMS issued QSO-23-14 which is the annual Report to Congress on the validation process CMS goes through with accrediting bodies to compare survey results. As has been the case for many years now CMS surveyors continue to find more condition level findings than do the accrediting bodies, particularly in the physical environment. The variance remains large and exceeds the goals established by Congress and CMS.
We have seen these significant variances for quite a few years now and we have anticipated that at some time we would start to see more condition level findings from the accrediting bodies in reaction to this annual report. We see more and more total findings in each report and the EC and LS findings we see on Joint Commission surveys far outnumber the number of clinical findings, but apparently CMS continues to find more at a condition level.
Given that this has been the case for multiple years we do not expect a knee jerk reaction or sharp uptick in Condition level findings. CMS has been considering alternatives to the current retrospective comparison of findings and they have proposed a concurrent validation model which may be the solution to the current disparity rates.
Post Pandemic Laboratory Changes:
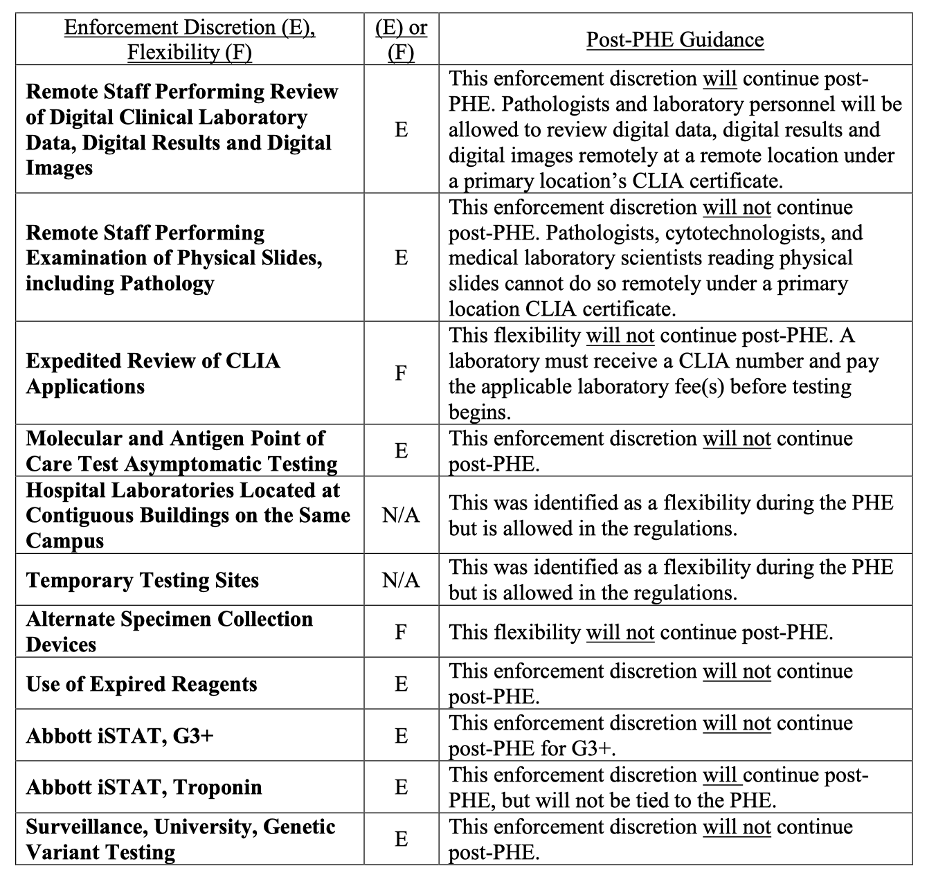
CMS also issued QSO-23-15 on May 11th discussing changes for laboratories post pandemic. For example, CMS has been requiring reporting of Covid test results as a result of the PHE. However, their authority to require that reporting lapsed with the PHE, so this reporting is no longer required. The CMS memo has a summary table, reproduced below that describes what ends and what continues after the PHE. The QSO memo also contains 20 FAQs of interest to laboratory providers.
CMS Summary Table of Post-PHE Enforcement Discretions and Flexibilities
Consultant Corner
Dear Readers,
Do you have your Orthopedic Disease Specific Care (DSC) certification? If you do, you are likely either 1) looking to maintain certification, 2) looking to build/expand your program, or 3) seeking recovery assistance. If you don’t, you might be looking to obtain initial certification since your community most likely serves this patient population.
Any step of the way, we can help! Learn more about our Orthopedic Certification Assistance service, meet your orthopedic specialist, and contact us today to schedule your expert consultation!
Thank You,
Jennifer Cowel, RN MHSA
JenCowel@PattonHC.com
Julia Finken, RN, BSN, MBA, CPHQ
julia.finken@hbsinc.com
Kurt Patton, MS RPh
Kurt@PattonHC.com
John Rosing, MHA
JohnRosing@PattonHC.com
