March 2023
Inside This Issue
 Racial and Ethnic Disparities in Pregnant and Postpartum Patients:
Racial and Ethnic Disparities in Pregnant and Postpartum Patients:
The lead article in Perspectives is rather complex in that it summarizes a January 17 Sentinel Event Alert #66 on racial and ethnic disparities in pregnant and postpartum patients. In addition it references a second document, Quick Safety #67 on Mental Health Conditions Causing Pregnancy Related Deaths.
The Sentinel Event Alert identifies the US as having the highest mortality rate for pregnant and postpartum patients among developed countries. There are references in the SE Alert with links and for this statistic it references a 17.4 mortality rate per 100,000 births in the US. This same reference displays a graphic with state-by-state mortality rates with Louisiana, Georgia, Indiana, and New Jersey having the highest mortality rates and California having the lowest.
The SE Alert also identifies some important ethnic comparisons from the CDC including:
- “Non-Hispanic black patients were 3 times more likely than white patients to die from pregnancy related causes.”
- “Native American pregnant patients were twice as likely to die than white pregnant patients.”
- “Black and Native American patients over the age of 30, have a mortality rate for pregnancy related causes that is 4-5 times higher than for white patients.”
- “Black pregnant patients with at least a college degree still have a mortality rate 5.2 times higher than white patients.”
The Sentinel Event Alert briefly discusses potential causes for these disparities and the Quick Safety provides a more in-depth analysis of mental health issues as a causative factor in a significant percentage of these deaths. The Quick Safety references another CDC data set that analyzed 36 state Maternal Mortality Review Committees analyses of just over 1,000 pregnancy related deaths and these committees identified almost 23% related to mental health conditions.
This is undoubtedly a complex issue, and you likely will want to have a team analyze these publications and data to determine how you might be able to enhance safety within your own patient population. We would also remind readers that the published Sentinel Event Alerts always contain recommendations for action, yet there is no standards-based mandate to implement each of these suggested actions.
There is however an expectation that accredited organizations will analyze the recommendations and implement those that might enhance safety for their particular organization, so you do want to document your careful analysis and some formal implementation efforts for those recommendations that are most meaningful to your organization.
Readers should also recall that TJC has new leadership standards in 2023 on reducing racial and ethnic healthcare disparities and these standards will become National Patient Safety Goals later this year. It is likely that this topic will be the subject of discussion by surveyors at the leadership and/or data use meetings.
If you provide maternal services, you will want to be able to address your analysis of these recommendations and more importantly your enhanced safety strategies as a result of your analysis. Organizations sometimes struggle to find a suitable issue to conduct the “proactive intensive analysis” TJC requires every 18 months. This may be an appropriate and meaningful issue to identify for potential analysis.

Collecting Data on Discrepancies Between Diagnoses:
This month’s Perspectives also mentions that some organizations have long-been confused about the Performance Improvement requirement to collect data on “all significant discrepancies between preoperative and post operative diagnoses.” The good news is TJC has posted a revision to PI.01.01.01, EP 4 that adds significant clarity to the requirement. The bad news is the older version that was confusing to some organizations may also have been unclear to surveyors because we can’t ever recall seeing this issue scored on a survey.
The newly revised version is both clearer and more specific and has been posted to the Joint Commission’s prepublication page on their website. The new version includes a note that states:
“The hospital’s medical staff determine which unexpected postoperative diagnoses are clinically significant. Examples may include, but are not limited to, the following:
- A preoperative pathology or cytology report interpreted as a malignancy, but no malignancy was found in the surgical specimen.
- A patient underwent surgery for acute appendicitis, but the appendix was normal in the postsurgical specimen.
- An operation was performed because of a presumed malignancy based on a radiology report, but no malignancy was found.”
Readers will want to work with their QAPI, medical staff, and surgery departments to ensure that there is a similar medical staff-developed definition for these discrepancies as well as an identification and PI reporting process to bring forward such discrepancies for analysis.
Titrations:
This month’s Consistent Interpretation column discusses MM.06.01.01, EP 3, which defines the verification process staff should go through j ust prior to medication administration. This EP has become one of the most frequently scored as a result of TJC’s focus on medication titrations.
ust prior to medication administration. This EP has become one of the most frequently scored as a result of TJC’s focus on medication titrations.
Specifically the EP requires staff to verify the product label and medication order, expiration date, examine for particulates, discoloration, or other loss of integrity, contraindications, that the medication is being administered at the proper time, in the prescribed dose, and by the correct route, and lastly that any concerns are discussed with the practitioner responsible for the treatment. We bolded the part of the EP that we see being scored most often as a result of the focus on titrations.
Prior to 2019 this EP was scored noncompliant much less often because it only required that the medication and its order match the product label. At that time the placeholder for scoring titration issues was PC.01.02.03, EP 3 for failure to perform reassessments as required. The Consistent Interpretation column this month sheds new light on how TJC is now carefully parsing the scoring on titration errors. They advise that surveyors score the MM standard is the medication order is not being followed. For example if a sedation titration should be adjusted if a certain RASS score is not being met. They also advise that the PC standard should be scored if there is a failure to document the physiologic parameter such as RASS when adjusting a sedation titration.
As luck would have it, the same day we saw this guidance published in Perspectives, we saw a 2023 survey report with both of these specific MM and PC standards scored noncompliant as a result of titrations. The MM standard was scored because a titration was adjusted even though a RASS score was documented that did not warrant an adjustment.
In addition the PC standards was scored noncompliant for another patient because a titration was adjusted and there was a failure to document any RASS score. The technical nuances of assigning fault to a specific EP, while interesting is likely not as important to our readers as is preventing either of these findings in your own organization. We continue to find sedation titrations most vulnerable to this type of scoring.
Blood pressure or pressor titrations are often better documented than sedation titrations which may be due to assistance by automated measurement and documentation systems which is not feasible with a RASS score. RASS requires staff to perform and document a specific and formal assessment. We do at times see gaps in policy requirements for RASS documentation that may account for some documentation gaps when staff are only recording RASS at a specific interval such as every 2 or 4 hours.
This interval-based documentation schedule may not align with assessments made at the time the titration needs to be adjusted. Another process gap we have seen is when staff assess the RASS as inadequate, adjust the titration rate, and then reassess and document the RASS is now where is should be, however the first assessment that was not on target is not documented. Organizations really need to think of the sedation titration documentation similar to pain medication.
With pain medication there is an assessment that indicates treatment is needed, followed by a reassessment after treatment to determine if the intervention was effective. Sedation titrations also need that assessment which indicates when an adjustment is needed, followed by a reassessment after the adjustment that documents if the target goal has been reached.
Discussion with ICU nurses during consults has identified many other clinical issues with sedation titrations such as observation of pain and blood pressure which may be resulting in titration rate adjustments outside of just the RASS parameter by itself. Unfortunately the authorization to change the rate may not be noted in the order details and the rationale why a change was made may also not be documented.
This month’s Consistent Interpretation column identified almost half of the hospitals surveyed last year received an RFI against this MM.06 standard. As there is also the potential for a “double ding” against the PC standard improvement of titration documentation is certainly warranted.
Getting timely documentation in an ICU setting may be difficult as urgent patient care needs take precedence, but organizations that have not yet considered TJC’s block charting option may want to give that another look. This would allow staff to “summarize” in a narrative note what adjustments took place and why during a 4-hour time block and avoid some of the minute-by-minute documentation gaps that are leading to RFIs.
 Medication Compounding for Home Care:
Medication Compounding for Home Care:
There are 8 months to go until the implementation of the new chapter. By now everyone should have obtained a copy and begun to formally analyze the workload, training, and environmental requirements. We noted the In Sight column on the last page of this month’s Perspectives mentions that TJC is working on revising the Medication Compounding Chapter for its Home Care Accreditation Manual.
While this chapter is not applicable at this time in the hospital accreditation manual, we do find the content and EP format very helpful to understanding TJC’s focus and requirements for sterile compounding. Unfortunately this home care chapter is not available to hospitals who do not also have a home care program.
The In Sight column informs us that TJC is working on incorporating all the new requirements from the 2023 version of USP Chapter 797 into a standard and EP format, which will be published in the revised medication compounding chapter for home care. TJC usually posts such new content to its Prepublication page on its website, and we would encourage hospitals with and without home care to download this chapter when it becomes available.
While USP is written in a narrative format, TJC will publish standards with specific elements of performance which can sometimes make it clearer as to what the actual requirement is.
 Accessing Medications at Night:
Accessing Medications at Night:
At the beginning of March we received an alert from TJC that a new FAQ on accessing medications at night, after the pharmacy is closed had been posted to their website. This is an issue that has been addressed in standards for many years in MM.05.01.13, EP 2 that describes a process for storing medications that might be needed at night “outside of the pharmacy.” In addition many more hospitals today have 24-hour pharmacy access than existed 30 years ago.
The new FAQ seems to reopen what seemed to be a resolved issue, by permitting access to the pharmacy by “trained prescribers and nurses designated by the organization and in accordance with Federal and State Law.” We have not heard what might have led to this change for an issue that appeared to be managed many years ago, but we can inform readers of some potential pitfalls.
We recall one situation, decades ago, where a hospital pharmacy was identifying medications as missing. They had a process that nursing leadership was permitted to enter the pharmacy at night, accompanied by a security officer who retained the key. Investigation identified that one of the security officers was entering the pharmacy unaccompanied by nursing. Corrective actions included routing the nighttime burglar alarm on/off report to the pharmacy director for review and validation with the nursing supervisors log of removed medications.
A second situation also decades ago involved another hospital pharmacy that permitted nighttime access, by nursing where one incident resulted in a survey by state authorities and the pharmacy director was cited for professional misconduct for authorizing anyone to enter the licensed pharmacy space without a pharmacist present. Bear in mind some state regulations view that pharmacy space in a hospital much like a community pharmacy where no one should be present in that space unless the pharmacist is present.
The oldest hospital standards manual we still have on file is from 2006, and the concept that nighttime access to medication storage should be outside of the pharmacy was already present in that edition. TJC did include advice in the new FAQ that after-hours access to the pharmacy by non-pharmacists should be minimized and eliminated as much as possible, however reopening the door to an antiquated practice seems unnecessary now.
The State Operations Manual from CMS tag A-0501 seems consistent with the prior requirement before this recent FAQ from TJC where CMS states:
“Medications must be available for administration to patients when needed, including when the pharmacy is not open. Methods to accomplish this when the pharmacy is not open could include, but are not limited to, one or more of the following: automated dispensing units outside the pharmacy, night cabinets, contracted services after hours via telepharmacy contracting, on-call pharmacists, etc.”
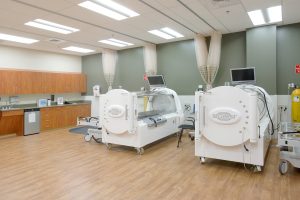 Hyperbaric Chamber Fire Drills:
Hyperbaric Chamber Fire Drills:
The lead article in this month’s EC News is about Hyperbaric Chamber Fire Drills. The basis for the fire drill requirements is derived from NFPA 99-2012, Chapter 14. You might remember in July 2022 TJC added a new element of performance #8 to EC.02.03.03 for hyperbaric chambers consistent with these NFPA requirements.
There is a note associated with this EP that details the differences in documentation of the fire drill in the hyperbaric area must include the time it takes to evacuate patients and staff using simulated extinguishment and evacuation procedures. Fire response procedures must also address the roles of the area staff.
We sometimes see hyperbaric services managed by the hospital, but more often managed by a contractor who specializes in this service. The contractors are usually very familiar with the NFPA requirements, but regardless of the use or non-use of a specialty contractor you want to ensure that documentation and fire safety plans are coordinated with your facilities leadership and files.
We would also suggest a review of the requirements of NFPA 99-2012, Chapter 14 in its entirety if you provide hyperbaric services as there are specific roles for a safety director, signage relative to fire safety, maintenance, and housekeeping. Joint Commission Resources has provided a link to one of their environmental checklists for hyperbaric services in the EC News article. We would encourage readers to collaborate with your facilities and hyperbaric service staff in completing this checklist.

Behavioral Health Crisis Units:
EC News also contains a helpful review of a developing new service called behavioral health crisis units. These are open space units specifically designed to meet the needs of behavioral health patients, which may be freestanding or adjacent to a hospital emergency room, and less often within a specific portion of an emergency department.
They usually provide personal recliner chairs for patients rather than beds or multiperson couches. Design features often include a more spacious and quieter environment that can be more soothing than the usual ED space. The Facilities Guideline Institute (FGI) has developed a guideline for the design of such units which can be downloaded from: https://fgiguidelines.org/wp-content/uploads/2022/06/FGI-Design-of-BHCUs_2022-06.pdf
 Hazard Vulnerability Analysis:
Hazard Vulnerability Analysis:
This month’s edition of EC News also contains a brief article on creating an executive summary of your Emergency Management hazard vulnerability analysis. The HVA is a requirement of the EM chapter to identify most likely and most risk-level emergency events that might befall your organization so that you can plan how to react and mitigate these more likely, higher risk events.
Many organizations already use the Kaiser Permanente designed HVA tool, but if you have not been using this you can download the tool from the California Hospital Association website using the following link: https://www.calhospitalprepare.org/post/updated-hva-tool-kaiser-permanente
 EM Chapter for Home Care:
EM Chapter for Home Care:
We should also remind readers who have a home care program that TJC has updated its EM Chapter for Home Care and that the new Chapter takes effect July 1, 2023. The format and changes to the chapter are similar to what hospitals did in 2022 as many standards and elements of performance are shared across programs. EC News this month does include a discussion on the changes for home care provided by their Project Director for Healthcare Standards Development and a JCR consultant that may be useful to your home care leadership team.
CMS did not issue any new QSO memos this past month.
Consultant Corner
Dear Readers,
Complying with regulations and achieving accreditations doesn’t need to be stressful with the right support. Please email any one of us below to schedule your mock or focus visit to best prepare your organization for a successful survey and safe patient care.
Jennifer Cowel, RN MHSA
JenCowel@PattonHC.com
Julia Finken, RN, BSN, MBA, CPHQ
julia.finken@hbsinc.com
Kurt Patton, MS RPh
Kurt@PattonHC.com
John Rosing, MHA
JohnRosing@PattonHC.com
