May 2023
Inside This Issue
 Ethical Issues Requirement:
Ethical Issues Requirement:
The May issue of Perspectives describes an update to LD.04.02.03, EP 1 on ethical issues in patient care that becomes effective August 27th, 2023. Normally if CMS is not mandating the change, TJC provides at least 6 months advance notice to get the new requirement implemented. However, this one seems like a minor wording change from the existing language.
At present the EP requires the “hospital to follow a process that allows staff, patients, and families to address ethical issues or issues prone to conflict.” The new language is being changed to say the “hospital develops and implements a process.” We looked at the standards manual to see if the current EP has a (D) for documentation and it does not. The Perspectives article format does not make it clear if a written, documented policy is required or not, and at present the official prepublication version is not yet posted to the TJC website as we are writing this. However, the new language stating “develop and implement” would make us think a written policy makes sense for consistency of staff implementation.
 Ventricular Assist Device (VAD) Implant Volume Reinstated:
Ventricular Assist Device (VAD) Implant Volume Reinstated:
During the pandemic and the official Public Health Emergency (PHE), CMS had eliminated the volume requirement for a minimum number of cases for the cardiothoracic surgeon VAD implant volume. The minimum volume expectation is going to restart effective May 11th when the PHE ends. CMS and TJC certification will expect each surgeon granted privileges to perform ventricular assist device implants to perform a minimum of 10 cases between May 11, 2023 and May 11, 2026. Organizations will want to factor that expectation into their decisions about privileges going forward.
 Use of Validated Tools for Suicide Risk Screening:
Use of Validated Tools for Suicide Risk Screening:
The May issue of Perspectives briefly describes the Quick Safety TJC published in March on the Use of Validated Tools for Suicide Risk Screening. The Quick Safety newsletter and Perspectives again make it clear that validated screening tools must not be altered as this may modify the reliability or accuracy of the tool.
Another concept that is made clear in both publications is that staff must be educated and competency assessed on the use of your chosen tool. If you look at NPSG.15.01.01, none of the EPs specifically describe the need for competency assessment on the use of a screening tool. Similarly, HR.01.06.01, EP 1 states that the hospital determines the competencies it requires. These bullet points in the Quick Safety and Perspectives would leave us to believe that competency assessment on the use of a screening tool is not an option.
Informed Consent Process:
 This month’s Consistent Interpretation column is on the informed consent process in RI.01.03.01, EPs 1 and 2. EP 1 requires the hospital to follow a written policy for informed consent and EP 2 requires the process to include a discussion about the planned care, potential benefits, likelihood of achieving goals, potential problems, risks, benefits, alternatives, and consequences of nontreatment.
This month’s Consistent Interpretation column is on the informed consent process in RI.01.03.01, EPs 1 and 2. EP 1 requires the hospital to follow a written policy for informed consent and EP 2 requires the process to include a discussion about the planned care, potential benefits, likelihood of achieving goals, potential problems, risks, benefits, alternatives, and consequences of nontreatment.
As usual the article does not provide insight on the appropriateness of the surveyor observations but does provide insight on TJC’s official position relative to scoring from their CITE database. Two pearls stand out on EP 1 from this secret database that likely are not general knowledge. The first pearl is that requiring patient dating and timing their signature on a consent form is not mandatory if not required by hospital policy. This could be documented only by the staff witness to the patient’s signature rather than the patient themselves.
The second pearl is that TJC does not themselves prohibit medical abbreviations on the consent form, this is instead determined by hospital policy. We have seen RFIs for many years for a failure of the patient to date and time a consent form and for the use of medical abbreviations used to describe the procedure. While you still may not want to use medical abbreviations from a risk management perspective, you might want to help avoid a “gotcha” by making the patient date and time their signature optional.
EP 2 which requires a discussion with the patient has one pearl in that the CITE database states that the organization determines where and/or how informed consent discussions are documented. Evidence that an informed consent discussion took place does not have to be on the consent form itself. Thus, policy could state that the physician could document that the informed consent discussion took place, information about risk and benefits, etc., was provided and questions were answered, etc., in the H&P, or documented on the consent for itself. Before modifying your consent process or form, do check your state regulations and your legal team, both may have more stringent requirements.
 New IC Standards?
New IC Standards?
The last page in Perspectives identifies that they will soon be doing a field review for new infection prevention standards. This is reported to be scheduled to be posted in the middle of this month. As this chapter is one of the most frequently scored chapters, we would recommend that readers be on the lookout for this field review and to be sure and respond to the field review questions that are posted. As we are writing this newsletter the draft standards have not yet been posted, but later you should see a link for field reviews on the TJC standards page of their website.
 Environmental Sustainability Standards:
Environmental Sustainability Standards:
The lead article in this month’s EC News is about the field review that has just concluded for their proposed environmental sustainability standards. We discussed the content of these proposed standards in our April newsletter and briefly discussed the limited nature of the field review questions. During the past month we have been seeing a wide array of comments on different discussion boards, including the ASHE discussion room. There appears to be many opinions both pro and con about these draft standards and it will be interesting to see how they evolve.
 ITM for Diagnostic Imaging Equipment:
ITM for Diagnostic Imaging Equipment:
There is a good EC News article on inspection, testing and maintenance of diagnostic imaging equipment. They detail the general requirement to adhere to manufacturer’s instructions for use and maintenance schedules, as well as some of the radiology specific EC standards requirements.
One specific suggestion they provide is for EC.02.04.01, EP 10 where the author advises that “organizations create a description of the quality control and maintenance procedures for advanced diagnostic imaging modalities.” We noted that the EP does not have a (D) for documentation but in rereading this EP, it certainly seems like this should be documented with a description and frequency.
EC.02.04.03 contains several very specific annual testing requirements for diagnostic imaging equipment with multiple very detailed criteria to evaluate in the test. We find that locating evidence of these tests and understanding the terminology used, which sometimes does not match the TJC element of performance, but rather uses a synonym, is often a struggle. We would strongly suggest keeping these required reports well organized and readily available with a translation table if synonyms are used.
The EC News article also provides a link to a 10 year summary of MRI related adverse events that was funded using federal monies, thus the article is in the public domain and you can access it using the following link: https://mriquestions.com/uploads/3/4/5/7/34572113/safety_review_2019_mp.13768.pdf
We would suggest sharing this EC News article with your radiology team and validating that your documentation is up to date, clear, understandable, adherent to MIFU, and accessible.
 Top Cited Physical Environment Standards:
Top Cited Physical Environment Standards:
The May issue of EC News contains an article on the most frequently scored environmental standards for the full year 2022. This is similar to our discussion from Perspectives last month but does contain more narrative details about the specific EC/LS standards that prove most challenging.
Environmental hazards in the behavioral health setting we all know are very frequently scored with a failure to perform a risk assessment being the second most frequently scored standard last year. This article provides links to the VA environmental risk assessment tool and the last article in the May EC News promotes a new JCR on Environment of Care Risk Assessment. This last article also provides a link to a JCR tool for conducting an assessment of the behavioral health environment.
The questions or issues to look for are very clearly written and thorough and the tool provides space to evaluate the relative risk of each hazard based on likelihood, scope and impact or severity. The document is in Word format and readers might want to consider converting the excellent questions into an Excel spreadsheet format to utilize formulas to help reach a conclusion on the relative risk of each identified hazard.
Regardless of which tool you use to evaluate suicide hazards in the behavioral health space you might want to verify that quality, facilities, and unit staff know of the existence of your environmental risk assessment and can locate a copy of the completed assessment. Too often people struggle to find their environmental risk assessments. We would also suggest being thorough with a low threshold to place a potential hazard on the evaluation tool.
Too often we find staff identify a theoretical hazard and, in their head, conclude its highly unlikely to ever be used by a patient for self-harm, so it never makes it onto the environmental risk assessment. Then the surveyor questions the presence of this potential hazard, and the fact that it is not on the risk assessment. Unfortunately, the surveyor’s perspective will be that you failed to consider the hazard, not that your team reached a conclusion about the hazard.
A key aspect is that risk assessments always need to be documented and not simply ignored because the hazard is deemed unimportant.
 Electrosurgery Safety:
Electrosurgery Safety:
EC News also has an interesting article on surgical fires and the risks with electrosurgery. The authors quote an ECRI study that states there are about 90-100 surgical fires each year leading to about 20 serious injuries and 1-2 patient deaths, and nearly 70% are associated with the use of electrosurgery devices.
The American Society of Gastrointestinal and Endoscopic Surgeons (SAGES) has developed an online training program designed to provide clinicians with greater insight on the devices mechanism of action, electrical features, relative energy, size of the electrode, waveforms, risks, and safety techniques. SAGES also has two versions of this training program, one for physicians operating the device and a second for operating room staff assisting in the case that has been endorsed by AORN.
End of the Public Health Emergency:
On May 1, CMS issued QSO 23-13 discussing the end of the public health emergency (PHE) for Covid. Readers may have heard of this on your local newscast much like ours which described this memo as addressing the end of the vaccine mandate for healthcare workers. That is not exactly true, and the memo does far more than that.
 The memo is divided up into provider types with sections for hospitals, SNFs, home care and hospice, ambulatory surgery, and others. During the pandemic CMS has issued many waivers, flexibilities, and enhanced payment systems. Each individual provider section describes the flexibilities that may continue and more frequently those that end. For those that end CMS clearly states, “the waiver ends upon conclusion of the PHE (May 11,2 023). We noted that for hospital providers the hospital at home program stands out as continuing until 12/31/24.
The memo is divided up into provider types with sections for hospitals, SNFs, home care and hospice, ambulatory surgery, and others. During the pandemic CMS has issued many waivers, flexibilities, and enhanced payment systems. Each individual provider section describes the flexibilities that may continue and more frequently those that end. For those that end CMS clearly states, “the waiver ends upon conclusion of the PHE (May 11,2 023). We noted that for hospital providers the hospital at home program stands out as continuing until 12/31/24.
Now the section on mandatory Covid vaccination is less clear, even though your local newscast may have described it as over. Take a careful look at the memo and you will note that it says the vaccine mandate will end “soon,” but unlike the rest of the memo it does not say it ends at the end of the PHE on May 11, 2023. So don’t stop yet on ensuring that new hires have their required vaccines, until such time that CMS places a date certain on the term “soon.”
NIOSH Managing Hazardous Medications:
In April NIOSH published its new guidance document on managing hazardous medications in healthcare facilities. You can access the document using the following link: https://www.cdc.gov/niosh/topics/hazdrug/
The new document updates the procedures and safety practices healthcare facilities should employ when handling hazardous medications since the last official publication in 2016. The list of medications considered hazardous has not been updated however and NIOSH has not yet made their 2020 list official. You will note when you go to the NIOSH site to download the new publication that it still contains links to the 2016 publication for the list of hazardous medications.
Section 3 of the new document discusses the requirement to develop an organization-specific list of hazardous medications in use. A starting point for developing that list is the NIOSH list from 2016 to identify known hazardous medications that you might be purchasing and using. In addition, there is an expectation that as you begin to use new medications they be evaluated to determine if they are hazardous but were not in existence or known to be hazardous back in 2016. This concept of creating a facility specific list is consistent with TJC standards approach and USP Chapter 800.
Section 4 then requires the organization to determine their exposure assessment. What procedures are being performed, what administrative controls are in place, what volumes of hazardous medication are being used and other factors.
Section 5 and 6 discuss the risk assessment and risk management processes Here is where you identify the risks you perceive as a result of your procedures and processes and control mechanisms. NIOSH has developed a graphic to help understand this aspect of the process.
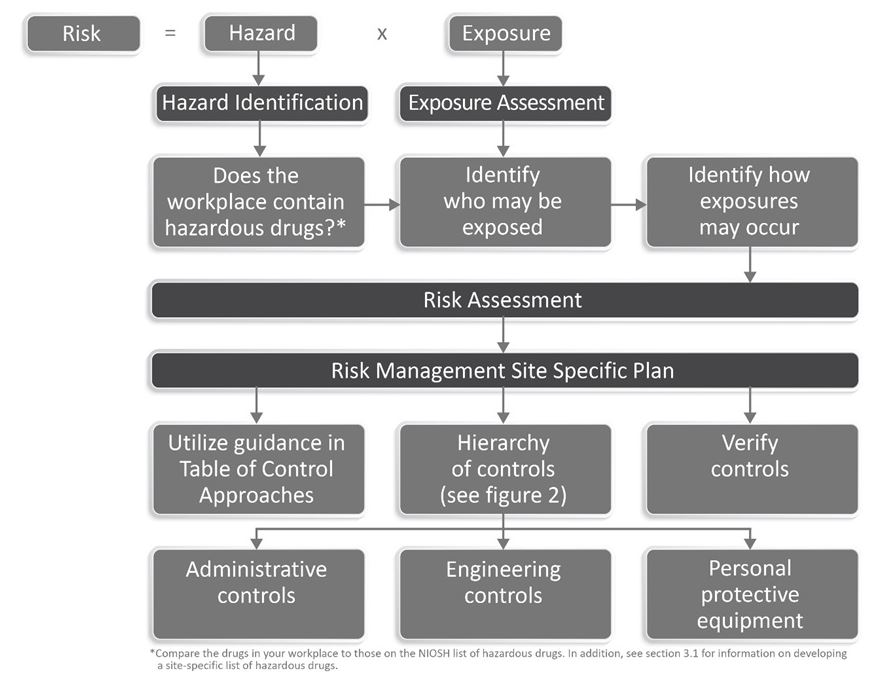 A key reference within the document is what they now call their “Table of Control Approaches for Safer Handling of Hazardous Drugs, by Activity and Formulation.” This is similar to the old Table 5 from the 2016 NIOSH document. NIOSH identifies activities such as receiving, transportation, compounding, administering, etc. and provides guidance of the required engineering controls (such as a biologic safety cabinet or CSTD) and personal protective equipment (such as gloves, gowns, respirator) workers need when performing these functions.
A key reference within the document is what they now call their “Table of Control Approaches for Safer Handling of Hazardous Drugs, by Activity and Formulation.” This is similar to the old Table 5 from the 2016 NIOSH document. NIOSH identifies activities such as receiving, transportation, compounding, administering, etc. and provides guidance of the required engineering controls (such as a biologic safety cabinet or CSTD) and personal protective equipment (such as gloves, gowns, respirator) workers need when performing these functions.
As you ramp up for implementation of USP Chapter 800 you will want to review this NIOSH document in detail to be sure that as you enhance worker safety from hazardous medications you incorporate guidance from both of these expert bodies.
Consultant Corner
Dear Readers,
We want to remind you of these two TJC survey announcements:
- 2-Year “Pull Forward” Survey: if your organization’s TJC triennial survey was delayed a year due to the public health emergency, your next survey may be pulled up to 2 years from your last. Please watch your intranet communications as you will receive a notification if you are pulled forward and contact your Account Executive with any questions.
- Health Care Equity Survey: even if you aren’t due for your TJC survey, you will still receive a 1-day remote survey for health care equity (Massachusetts only).
Don’t be unprepared when the survey team arrives! Please contact us below or call 888-PHC-INC1 to best prepare your organization for a successful survey.
Have a Wonderful Month,
Jennifer Cowel, RN MHSA
JenCowel@PattonHC.com
Julia Finken, RN, BSN, MBA, CPHQ
julia.finken@hbsinc.com
Kurt Patton, MS RPh
Kurt@PattonHC.com
John Rosing, MHA
JohnRosing@PattonHC.com
