November 2023
Inside This Issue
 2024 ORYX Reporting Requirements:
2024 ORYX Reporting Requirements:
The most important news in this month’s issue of Perspectives is the article on the 2024 ORYX Reporting Requirements. We have to admit, the announcement was somewhat confusing to us as it includes information about what’s new in ORYX, what’s discontinued, what’s available to choose from, and what is mandatory.
The last sentence in the article contains a link to the Joint Commission’s ORYX Performance Measurement Page and there you can find a 2024 and a 2023 grid that does a good job of explaining what was required in 2023 and what now is required for 2024 chart abstracted measures, electronic quality measures (eCQM), and what external data TJC wants you to provide them access to. The first column on the grid details the universe of hospitals with mandatory ORYX requirements and is divided into 5 categories:
- Large hospitals with ≥ 26 licensed beds or ≥ 50,000 outpatient visits + obstetrical services
- Large hospitals with ≥ 26 licensed beds or ≥ 50,000 outpatient visits + NO obstetrical services
- Small hospitals with < 26 licensed beds and < 50,000 outpatient visits
- Critical access hospitals
- Freestanding psychiatric hospitals
At this time there are also 5 categories of hospitals that are exempt from ORYX reporting requirements, and these include:
- Free standing Children’s Hospitals
- Long term care acute hospitals (LTACH)
- Inpatient rehabilitation hospitals (IRF)
- HCOs in the PPS exempt Cancer Hospital Quality Reporting Program (PCHQR)
- Indian Health Service/Tribal hospitals
The next 3 columns on the grid then explain the aforementioned 2024 requirements for chart abstracted measures, electronic clinical quality measures, and external data sources that Joint Commission wants you to provide them access to. For example, most of our readers will be in the “large” category of hospitals with 26 or more beds, or 50,000 or more outpatient visits. For those large hospitals which have obstetrical services there is only one required chart abstracted measure and that is PC-06 which is “unexpected complications in term newborns.” If you don’t have obstetrical services, there are no required chart abstracted performance measures.
If you look at the 2023 grid you will note that the current year requirement for PC-01, PC-02, and PC-05 goes away next year. It is also possible that your hospital may be able to capture and report PC-06 as an electronic clinical quality measure and if you can do this, you have no requirement for chart abstracted measures and your ability to report PC-06 as an eCQM, counts as one of the 3 self-selected eCQMs that must be reported. Large hospitals with obstetrical services must report ePC-02, ePC-07, and Safe Use of Opioids as mandatory eCQMs. In addition, these large hospitals with obstetrical services must also choose 3 additional eCQMs from the menu of optional measures. In 2024 there are two new optional measures, one, the global malnutrition composite score and the second, on hospital harm-opioid related adverse events.
Small hospitals and critical access hospitals must submit any 3 performance measures applicable to their patient population and these can be either chart abstracted or electronic quality measures. Freestanding psychiatric hospitals must report chart abstracted HBIPS -2 and 3, as they did in 2024, as well as any one additional self-selected chart abstracted measure available from a menu of 6 available chart abstracted measures. There are two new options for chart abstracted measures in 2024 including screening for social drivers of health and screen-positive rate for social drivers of health.
The one new requirement that is applicable to all categories of hospitals is to “participate” in the Joint Commission NHSN group for data you are already submitting to NHSN. The term participate means you will allow the Joint Commission to access the de-identified data you have submitted to the CDC’s NHSN project. The Perspectives article contains a brief description on this access process and TJC will be notifying organizations with details on how to grant this access later this year or early in 2024.
Again, understanding the specifics of these requirements is complex, but if you access the 2023 and 2024 grids mentioned in the Perspectives article from the TJC website, and look at them side by side for your hospital type, it will help further your understanding of these evolving requirements.
 Certification Document Upload:
Certification Document Upload:
Perspectives also contains a piece of new information about the document upload process for required documents that must be submitted for a certification review. Currently organizations anticipating a certification review upload required documents to a SharePoint site. It is anticipated that this process will change in January when document upload will be done directly on the Joint Commission Connect site.
Managing Complaints:
 This month’s Consistent Interpretation column discusses RI.01.07.01 which deals with complaint management. The TJC scoring frequency for this standard and its elements of performance is extraordinarily small. However, as healthcare organizations know, CMS often performs a more exacting scrutiny of complaint and grievance management than Joint Commission appears to.
This month’s Consistent Interpretation column discusses RI.01.07.01 which deals with complaint management. The TJC scoring frequency for this standard and its elements of performance is extraordinarily small. However, as healthcare organizations know, CMS often performs a more exacting scrutiny of complaint and grievance management than Joint Commission appears to.
Looking at the massive Excel spreadsheet of complaint scoring data that CMS shares on its website, we noted just under 1500 observations of noncompliance with CMS tags A-0118 – A-0122. We also noted the guidance to surveyors section of this month’s Consistent Interpretation column frequently mentions the Joint Commission does not require a policy, it requires only a “process” and it requires surveyors to evaluate adherence to that process. Expecting staff to consistently carry out a process without written policies and procedures seems error prone, and more importantly, it is inconsistent with CMS’ interpretive guidance.
Our advice on this month’s column is to read the column and then read the CMS State Operations Manual, tags A-0118 – A-0122 and to use both the TJC and CMS references to gauge your state of readiness. In tag A-0118 it specifically advises CMS surveyors to examine if: “The hospital is following its grievance policies and procedures.” As this is an issue with intense CMS focus, you want to be ready for either group to evaluate your compliance, and on this particular issue CMS seems to have a more intense and frequent focus.
Prevention of Surgical Fires:
I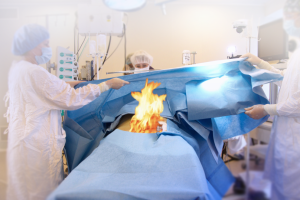 n October, TJC published an updated Sentinel Event Alert #68 on Prevention of Surgical Fires. This had initially been covered in an alert issued back in 2003. TJC has removed the 2003 alert from its website, but we had a copy on file, and it is impressive to see how much more detailed the new alert is on the explanation of risks and the depth of the recommendations. The risk element is the so-called fire triangle of oxidizing agents (oxygen) plus electrosurgical devices (heat/ignition), plus alcohol skin-based preparations (fuel) or other fuel sources.
n October, TJC published an updated Sentinel Event Alert #68 on Prevention of Surgical Fires. This had initially been covered in an alert issued back in 2003. TJC has removed the 2003 alert from its website, but we had a copy on file, and it is impressive to see how much more detailed the new alert is on the explanation of risks and the depth of the recommendations. The risk element is the so-called fire triangle of oxidizing agents (oxygen) plus electrosurgical devices (heat/ignition), plus alcohol skin-based preparations (fuel) or other fuel sources.
There are 6 specific recommendations published in the alert and you will want your surgical teams to evaluate your current status regarding these recommendations and consider modifying procedures where gaps are noted. In addition, for those of you anticipating survey in the next few months, we would encourage you to be sure to distribute the Alert to the surgical team and begin the process of analysis so that surgical team members that might be asked about this Alert are in a position to reply that “it has been distributed to our entire surgical team and we are in the process of performing a detailed analysis to determine if we have opportunities to improve.”
 Environmental Standards in Critical Access Hospitals:
Environmental Standards in Critical Access Hospitals:
This month’s issue of EC News has a review article on difficult environmental standards in critical access hospitals, and a summary of the presentation their Director of Physical Environment delivered at this year’s Executive Briefings sessions. In recent months EC News has done a good job on informing readers about the most frequently scored standards and EPs in the physical environment. We too have briefly summarized much of this content, but the frequency of scoring these same standards year after year remains extremely high.
These two new articles are certainly worth sharing with your EC team, but the key is getting a handle on where your organization stands and what your organization is going to do about potential vulnerabilities. For example, you might want to consider the following questions in your review of the top 5 or top 10 most frequently scored standards.
- What is our team’s opinion on our level of risk of having this same standard scored non-compliant in our organization?
- Was this standard scored noncompliant on our last TJC survey?
- What data is currently available that provides us some confidence that we are mostly or fully compliant with this issue today?
- Who performs this analysis that provides us this data?
- What is the numerator and denominator that gave us this compliance perspective?
- What percentage of our entire organization is captured in that denominator?
- How often do we perform this audit?
- Has our self-assessment been validated by either internal leadership rounds or external consultants?
- If our compliance is perceived to be high, show me the nearest 3 locations that validate this perception.
- If our compliance is perceived to be low, what do we need to do to eliminate this risk?
 Winter and Grounds Maintenance:
Winter and Grounds Maintenance:
Now that summer is over and we can stop complaining about the heat, it is unfortunately time to consider the arrival of winter and preparation for what is to come, and for us to begin complaining about the cold. EC News has a good article to review on preparing for winter grounds maintenance. The authors advise making two lists, one of routine preventative maintenance activities such as painting doors and repairing locks, and a second list of tasks that need to be implemented when you get that first 2-inch snowfall.
EC News also provides some advice on managing external contractors and making your requirements clear to them. Unfortunately, given current inflation, they also warn that you might be anticipating additional costs in this regard. The article provides advice from the University of Rochester Medical Center about tracking slips, trips, and falls both inside and outside the hospital as a key performance indicator to help analyze how effective your snow removal procedures are. This article is also tied to one of their periodic Checklists, a 4 pager this month that can be used for an in-depth analysis of your preparation for winter and snow removal.

New USP Chapters:
November 1st has arrived and the new Chapters 795, 797 and 800 are official. TJC has indicated that they will begin to survey to these new requirements in January. State Boards of Pharmacy will have their own unique timelines depending on if they reference, “most current edition” or a specific version in their regulations. We have seen a draft of the new TJC surveyor evaluation tool for Chapters 797 and 800. While it has not yet been officially published by TJC, we would anticipate this sometime before January.
We had a question this past month from a client asking about the optional “shoulds” in the new chapters, and in particular the “should” in section 13; Compounding in USP Chapter 800. This section advises use of a plastic-backed mat being used on the work surface of the PEC to collect any spillage or trace hazardous drug contamination. The questioner stated that these were expensive and had to be replaced during the day and immediately if visibly contaminated. Our advice was to use a traditional risk assessment format as an aide in decision making. The process would be similar to the process you would use if you were evaluating an environmental or infection related hazard.
First you would want to evaluate how extensive your hazardous drug compounding is. Do you have a large oncology service where lots of antineoplastic medications are prepared every day, or is it more sporadic to a few a month? We also advised taking a look at what the organization is learning from its environmental wipe sampling. This is described in section 6 of USP Chapter 800 and unfortunately it too is an optional “should,” but it may provide insight into risk. You might notice trace contamination in your PEC or in the area immediately adjacent to PEC.
However, you might identify trace contamination in other parts of the SEC, Anteroom, pass-through, or even in the main pharmacy. The wider the contamination, the greater the risk and potentially greater value to using the plastic backed pads during compounding. Another important consideration in your risk assessment is to use the professional literature and multidisciplinary team members expertise in evaluating the information.
The traditional risk assessment format and the terminology is over-used in The Joint Commission process, but it can be a valuable aide in decision making and protecting your organization. We should also point out that the process we are describing here is somewhat the inverse of what USP Chapter 800 describes in section 2 called an assessment of risk. This assessment of risk process is used to determine if there are routine and mandatory containment strategies that you might waive for a specific drug and dosage form, because the risk of contamination is very low due to that dosage form not requiring any manipulation. For example, a unit dose packaged oral tablet that is not going to be crushed or split. In this Chapter 800 evaluation of risk approach, you are specifically identifying a medication and dosage form that can use fewer required safety precautions and this evaluation must be refreshed every 12 months.
In the coming years, surveyors are going to get used to seeing some very good practices, many large organizations are implementing almost all of the optional “shoulds” and over time it may become a routine expectation that is noticed if one or more of the “shoulds” is not in place in a similarly large volume oncology service. When we reach that level of familiarity with the requirements, the documented risk assessment might be helpful to demonstrate why you chose not to, what literature you used and who helped make that decision.
It is also likely that some employees will experience their own malignancies later in life, and regardless of causation, they will question if on-the-job exposure was an important factor. If you did not implement optional requirements such as medical surveillance, or these optional pads, in a high-volume organization just because it was expensive to do, your organization may be perceived as having greater responsibility in causation as compared to an organization that conducted a formal multidisciplinary risk assessment analysis to help guide decision making.
 New Survey Activity Guide:
New Survey Activity Guide:
The Joint Commission published a new version of their customer Survey Activity Guide in October. The guide includes a summary of what’s new and it appears that the kitchen tracer tool has been updated as well as some references to their new 3-year privileging cycle for practitioners with privileges.
CMS did not publish any new QSO memo’s this past month.
Consultant Corner
Dear Readers,
As we enter the Holiday Season and start to bundle up, we would like a moment to give gratitude to our loyal
subscribers and for your passion to provide the safest and highest quality of patient care. Because of you, healthcare
is a better place. We are grateful and appreciative for each and every one of you!
Thank You,
Jennifer Cowel, RN MHSA
JenCowel@PattonHC.com
Julia Finken, RN, BSN, MBA, CPHQ
julia.finken@hbsinc.com
Kurt Patton, MS RPh
Kurt@PattonHC.com
John Rosing, MHA
JohnRosing@PattonHC.com
